Abstract
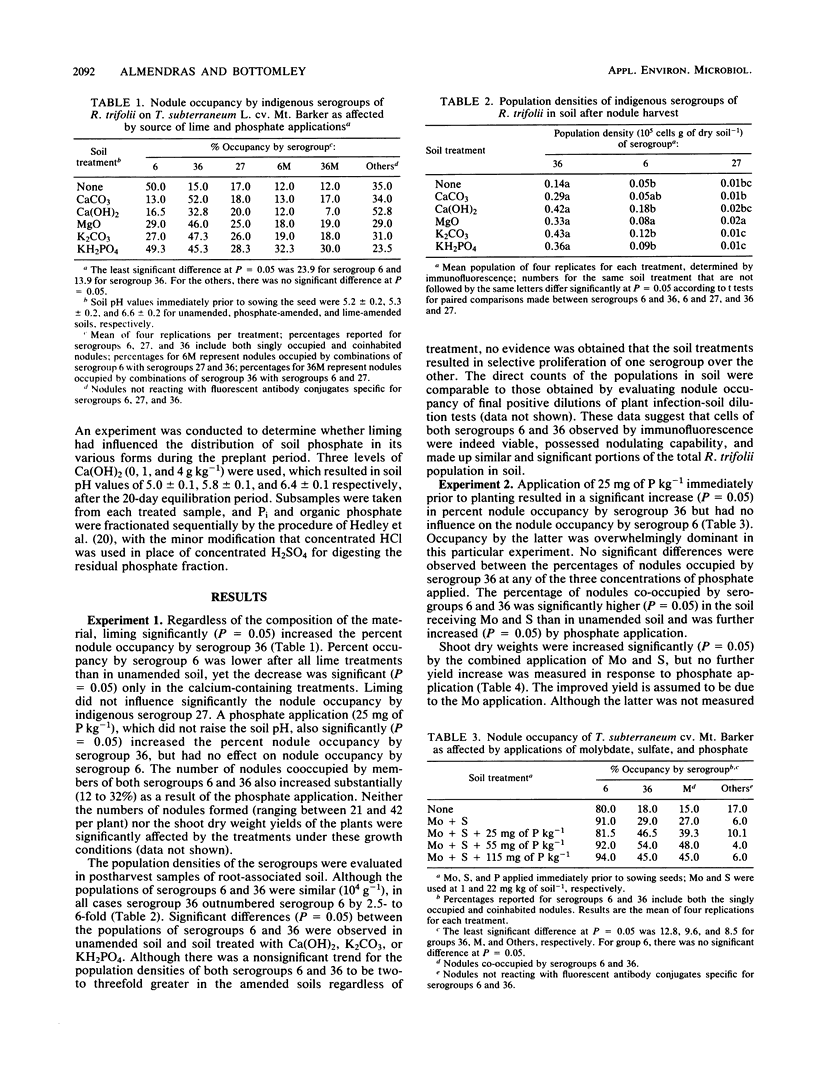
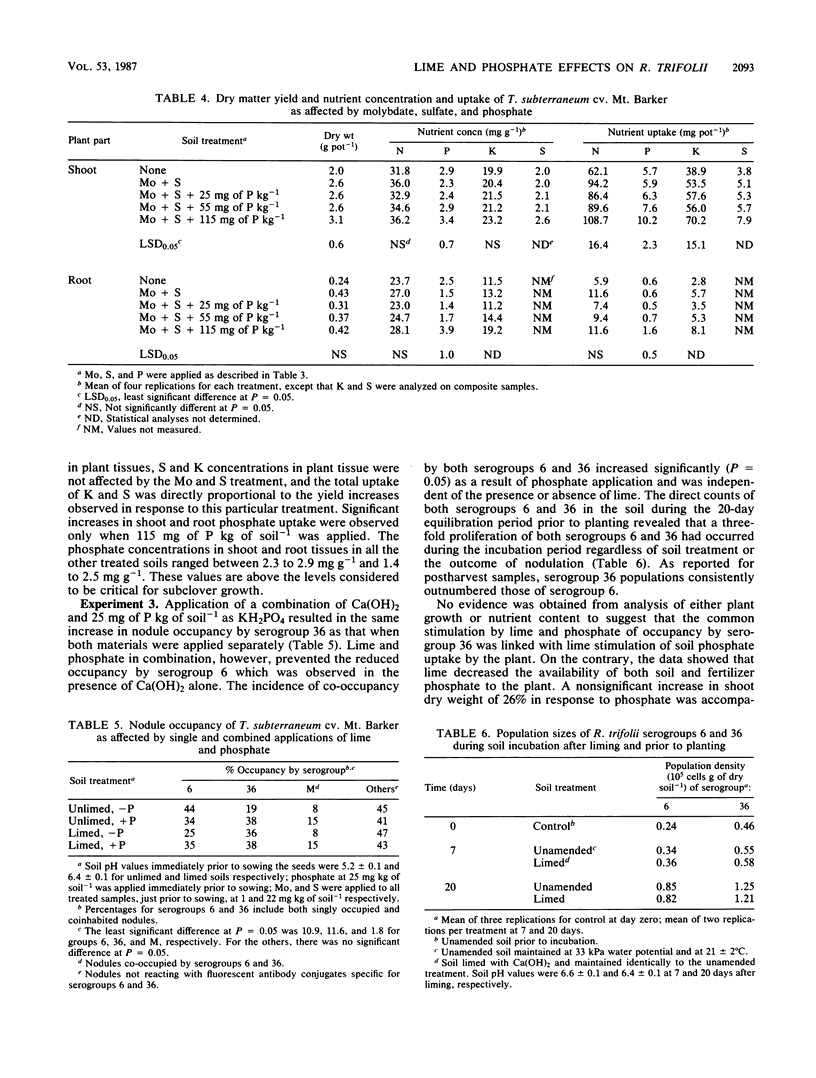
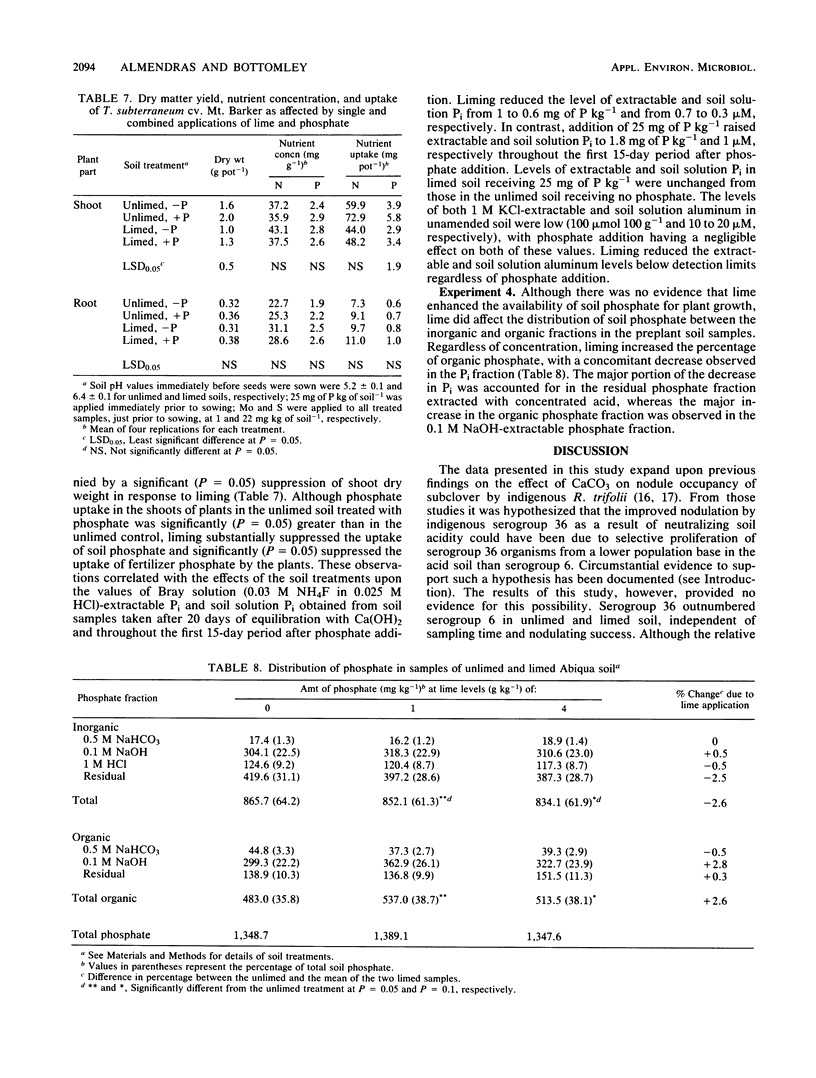
Previous research had identified four serogroups of Rhizobium trifolii indigenous to the acidic Abiqua soil (fine, mixed, mesic Cumulic Ultic Haploxeroll). Nodulation of subterranean clover (Trifolium subterraneum L.) by two of the serogroups, 6 and 36, was differentially influenced by an application of CaCO3 which raised the pH of the soil from 5.0 to 6.5. These studies were designed to characterize this phenomenon more comprehensively. Liming the soil with either CaCO3, Ca(OH)2, MgO, or K2CO3 significantly (P = 0.05) increased the percent nodule occupancy by serogroup 36, whereas the percent nodule occupancy by serogroup 6 was decreased, but the decrease was significant (P = 0.05) only after application of either CaCO3 or Ca(OH)2. Application of KH2PO4 (25 mg of P kg of soil−1), which did not change soil pH, also significantly (P = 0.05) increased the percent nodule occupancy by serogroup 36. Application of KH2PO4 in combination with Ca(OH)2 produced the same increase in nodule occupancy by serogroup 36 as did individual application of the two materials. Soil populations of serogroup 36 consistently, and in the majority of cases significantly (P = 0.05), outnumbered those of serogroup 6 before planting and after harvest regardless of soil treatment or the outcome of nodulation. Soil chemical and plant analyses provided no evidence that liming was simulating phosphate addition by increasing the availability and subsequent uptake of soil Pi by the subclover plants. Liming did, however, result in a significant transformation (30 to 50 mg of P kg of soil−1) of Pi from the residual soil Pi fraction into an NaOH-extractable organic P fraction during the preplant equilibration period.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demezas D. H., Bottomley P. J. Autecology in Rhizospheres and Nodulating Behavior of Indigenous Rhizobium trifolii. Appl Environ Microbiol. 1986 Nov;52(5):1014–1019. doi: 10.1128/aem.52.5.1014-1019.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dughri M. H., Bottomley P. J. Effect of Acidity on the Composition of an Indigenous Soil Population of Rhizobium trifolii Found in Nodules of Trifolium subterraneum L. Appl Environ Microbiol. 1983 Nov;46(5):1207–1213. doi: 10.1128/aem.46.5.1207-1213.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Bottomley P. J. Influence of Phosphate on the Growth and Nodulation Characteristics of Rhizobium trifolii. Appl Environ Microbiol. 1987 Sep;53(9):2098–2105. doi: 10.1128/aem.53.9.2098-2105.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther W. L., Loneragan J. F. Calcium and Nodulation in Subterranean Clover (Trifolium subterraneum L.). Plant Physiol. 1968 Sep;43(9):1362–1366. doi: 10.1104/pp.43.9.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S. N., Bohlool B. B. Competition Among Rhizobium leguminosarum Strains for Nodulation of Lentils (Lens esculenta). Appl Environ Microbiol. 1983 Mar;45(3):960–965. doi: 10.1128/aem.45.3.960-965.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



