Abstract
Several cellular interactions have been identified as potentially important in fibrogenesis induced by mineral dusts. Evaluation of their relative importance in vivo remains a problem. Sealed diffusion chambers limited by Nuclepore membranes and implanted into mouse peritoneal cavities provide a means of assessing different stages of fibrogenesis by separating initiating mechanisms (dust-macrophage-lymphocyte combinations inside the chamber) from the target tissue. Fibrous reactions surrounding the chambers were quantitated by macroscopic and histological scoring, and by measurement of 14C glycine incorporated at the reaction site. Using this model the fibrogenicity of Rhodesian A chrysotile asbestos, DQ12 quartz and haematite were compared. Whereas asbestos-macrophage ratios of between 6 . 6 and 900 micrograms/10(6) mouse peritoneal macrophages (MPM) produced fibrosis, an equivalent response was obtained with 0.05 micrograms silica/10(6) MPM. Silica in amounts greater than this produced macrophage cytotoxicity without fibrogenesis. Haematite-macrophage combinations produced no significant fibrosis. It was confirmed that a direct dust-macrophage interaction forms the essential first step in fibrogenesis by both asbestos and silica and that the fibrogenicity is mediated by diffusible factor(s). Prior stimulation of host mice with Freund's complete adjuvant modified the fibrogenic response to some dust-cell combinations, suggesting an important role for host responses in determining the outcome of fibrogenic stimuli.
Full text
PDF



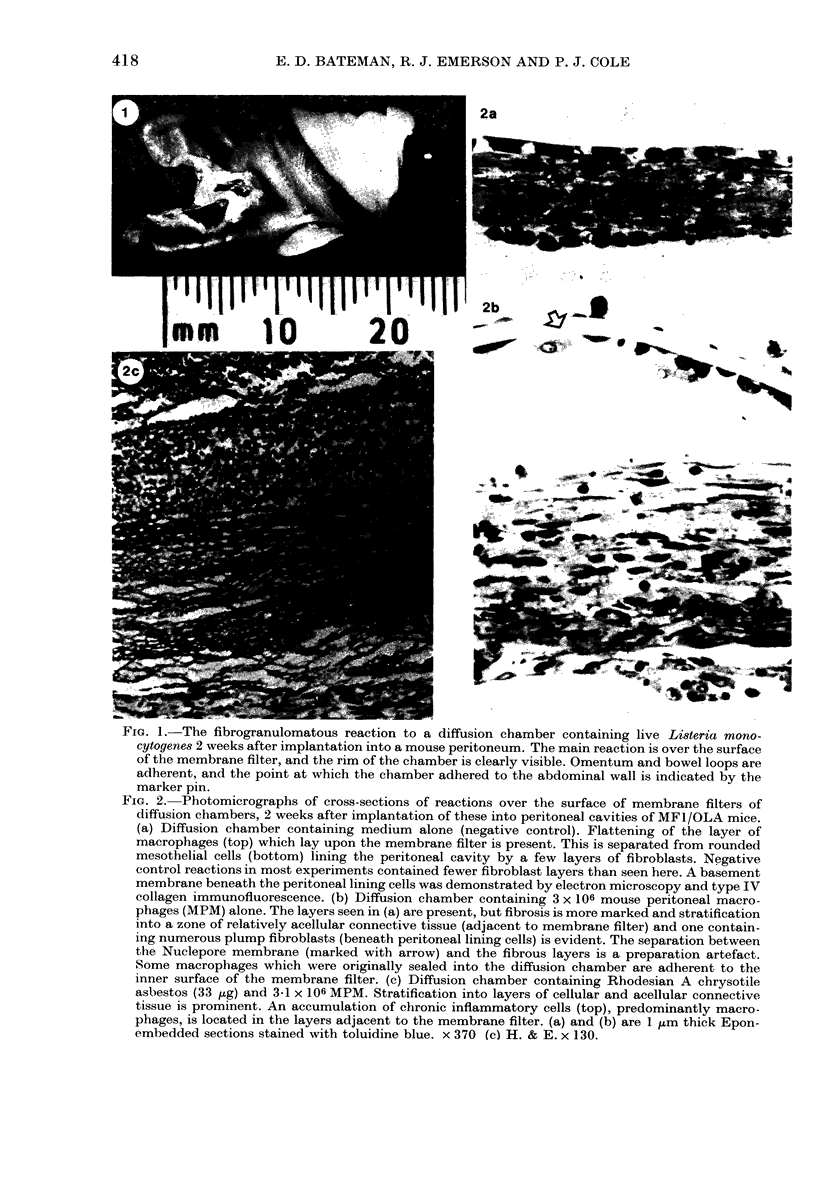







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalto M., Kulonen E. Fractionation of connective-tissue-activating factors from the culture medium of silica-treated macrophages. Acta Pathol Microbiol Scand C. 1979 Jun;87C(3):241–250. [PubMed] [Google Scholar]
- Aalto M., Potila M., Kulonen E. The effect of silica-treated macrophages on the synthesis of collagen and other proteins in vitro. Exp Cell Res. 1976 Jan;97:193–202. doi: 10.1016/0014-4827(76)90668-6. [DOI] [PubMed] [Google Scholar]
- Aalto M., Turakainen H., Kulonen E. Effect of SiO2-liberated macrophage factor on protein synthesis in connective tissue in vitro. Scand J Clin Lab Invest. 1979 May;39(3):205–213. doi: 10.1080/00365517909106095. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman E. D., Turner-Warwick M., Adelmann-Grill B. C. Immunohistochemical study of collagen types in human foetal lung and fibrotic lung disease. Thorax. 1981 Sep;36(9):645–653. doi: 10.1136/thx.36.9.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell R., Anderson M. The induction of fibrogenesis by silica-treated alveolar macrophages. Environ Res. 1973 Dec;6(4):389–394. doi: 10.1016/0013-9351(73)90054-6. [DOI] [PubMed] [Google Scholar]
- CURRAN R. C., ROWSELL E. V. The application of the diffusion-chamber technique to the study of silicosis. J Pathol Bacteriol. 1958 Oct;76(2):561–568. doi: 10.1002/path.1700760225. [DOI] [PubMed] [Google Scholar]
- Chamberlain M., Brown R. C., Davies R., Griffiths D. M. In vitro prediction of the pathogenicity of mineral dusts. Br J Exp Pathol. 1979 Jun;60(3):320–327. [PMC free article] [PubMed] [Google Scholar]
- Göthe C. J., Swensson A. Effect of BCG on lymphatic lung clearance of dusts with different fibrogenicity. An experimental study on rats. Arch Environ Health. 1970 May;20(5):579–585. doi: 10.1080/00039896.1970.10665665. [DOI] [PubMed] [Google Scholar]
- Hamilton J., Vassalli J. D., Reich E. Macrophage plasminogen activator: induction by asbestos is blocked by anti-inflammatory steroids. J Exp Med. 1976 Dec 1;144(6):1689–1694. doi: 10.1084/jem.144.6.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppleston A. G., Styles J. A. Activity of a macrophage factor in collagen formation by silica. Nature. 1967 Apr 29;214(5087):521–522. doi: 10.1038/214521a0. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., DeStefano M. J. Use of the local anesthetic lidocaine for cell harvesting and subcultivation. In Vitro. 1975 Nov-Dec;11(6):379–381. doi: 10.1007/BF02616374. [DOI] [PubMed] [Google Scholar]
- Richerson H. B., Seidenfeld J. J., Ratajczak H. V., Richards D. W. Chronic experimental interstitial pneumonitis in the rabbit. Am Rev Respir Dis. 1978 Jan;117(1):5–13. doi: 10.1164/arrd.1978.117.1.5. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. H., Siddique I. H., Williams B. B. In vitro effects of listerial hemolysin on rat brain mitochondria. Am J Vet Res. 1976 Jun;37(6):735–736. [PubMed] [Google Scholar]
- duBuy H. Effect of silica on virus infections in mice and mouse tissue culture. Infect Immun. 1975 May;11(5):996–1002. doi: 10.1128/iai.11.5.996-1002.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



