Abstract
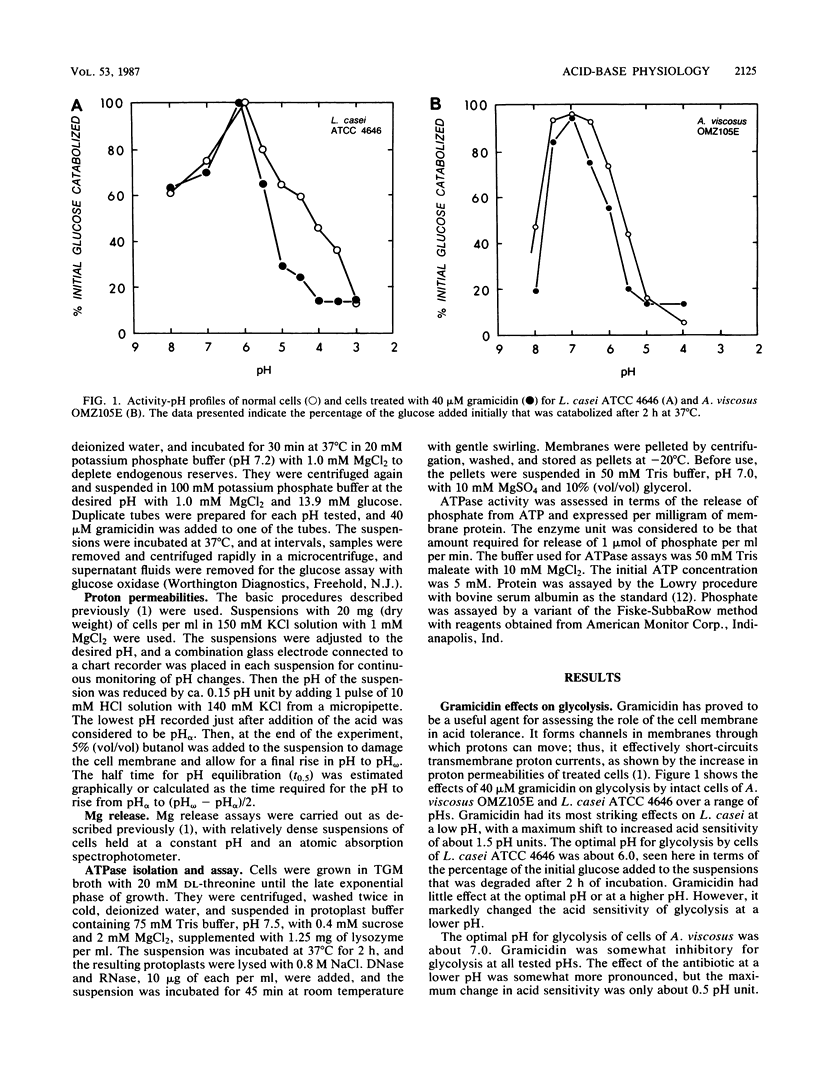
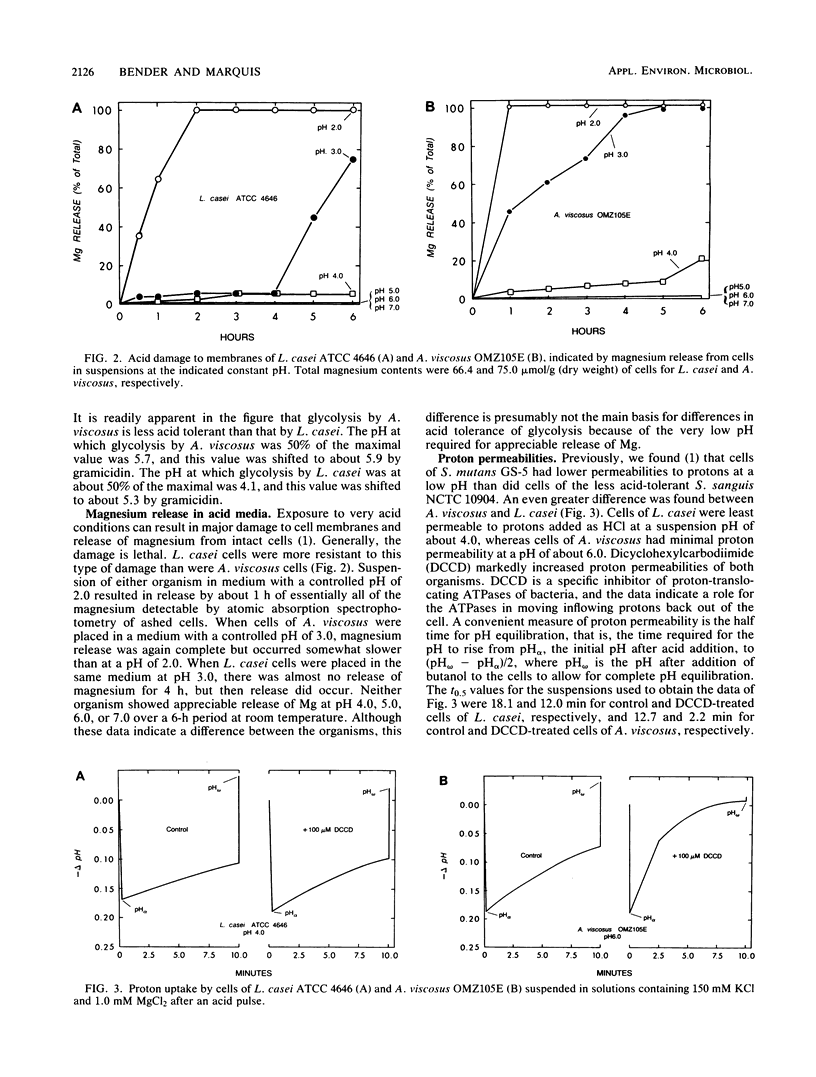
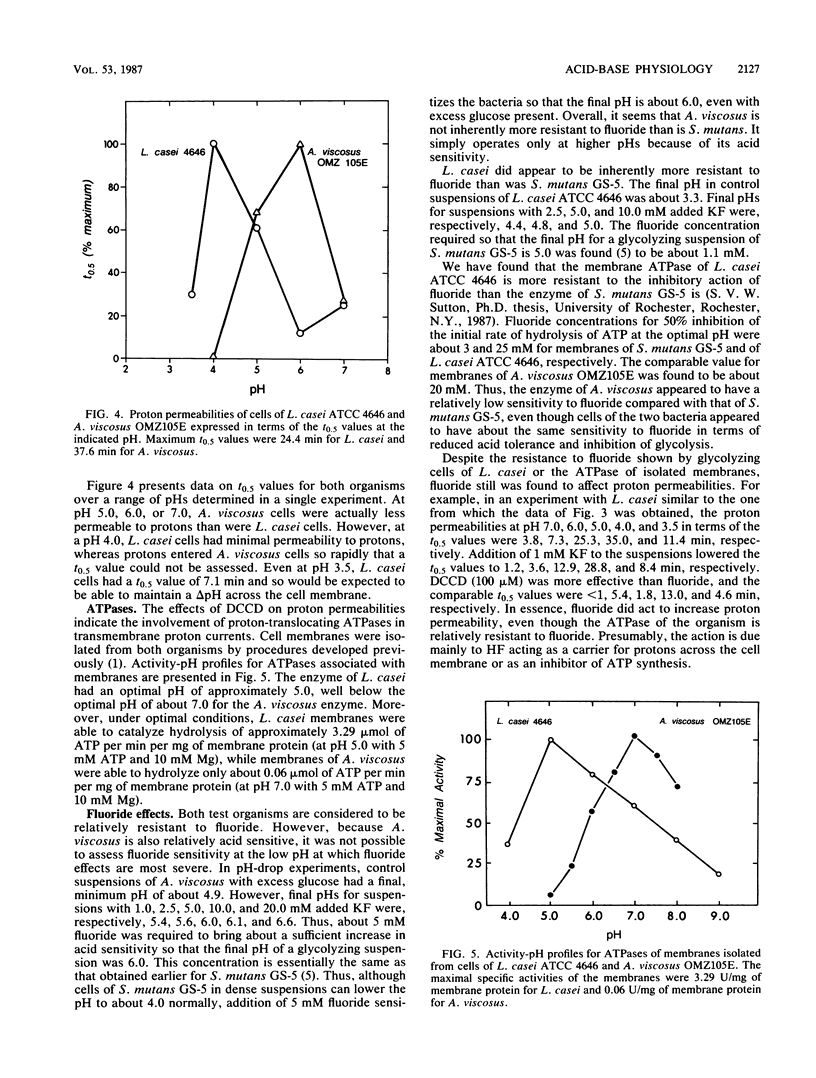
Lactobacillus casei ATCC 4646 and Actinomyces viscosus OMZ105E were found to differ markedly in acid tolerance. For example, pH profiles for glycolysis of intact cells in dense suspensions indicated that glycolysis by L. casei had an optimal pH of about 6.0 and that glucose degradation was reduced by 50% at a pH of 4.2. Comparable values for A. viscosus cells were at pHs of about 7.0 and 5.6. The difference in acid tolerance appeared to depend mainly on membrane physiology, and the addition of 40 microM gramicidin to cell suspensions increased the sensitivity of the glycolytic system by as much as 1.5 pH units for L. casei and up to 0.5 pH unit for A. viscosus. L. casei cells were inherently somewhat more resistant to severe acid damage than were A. viscosus cells, in that Mg release from L. casei cells in medium with a pH of 3.0 occurred only after a lag of some 4 h, compared with rapid release from A. viscosus cells. However, the major differences pertinent to the physiology of the organisms appeared to be related to proton-translocating ATPases. Isolated membranes of L. casei had about 3.29 U of ATPase per mg of protein, compared with only about 0.06 U per mg of protein for those of A. viscosus. Moreover, the ATPase of L. casei had a pH optimum for hydrolytic activity of about 5, compared with an optimal pH of about 7 for that of A. viscosus.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender G. R., Sutton S. V., Marquis R. E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986 Aug;53(2):331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender G. R., Thibodeau E. A., Marquis R. E. Reduction of acidurance of streptococcal growth and glycolysis by fluoride and gramicidin. J Dent Res. 1985 Feb;64(2):90–95. doi: 10.1177/00220345850640021701. [DOI] [PubMed] [Google Scholar]
- Eisenberg A. D., Bender G. R., Marquis R. E. Reduction in the aciduric properties of the oral bacterium Streptococcus mutans GS-5 by fluoride. Arch Oral Biol. 1980;25(2):133–135. doi: 10.1016/0003-9969(80)90089-8. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Onose H. pH measurements of Actinomyces viscosus colonies grown on media containing dietary carbohydrates. Arch Oral Biol. 1978;23(2):105–109. doi: 10.1016/0003-9969(78)90146-2. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Bowden G. H. Response of freshly isolated strains of Streptococcus mutans and Streptococcus mitior to change in pH in the presence and absence of fluoride during growth in continuous culture. Infect Immun. 1982 Apr;36(1):255–262. doi: 10.1128/iai.36.1.255-262.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Boyar R. M., Bowden G. H. Influence of pH and fluoride on properties of an oral strain of Lactobacillus casei grown in continuous culture. Infect Immun. 1985 Jun;48(3):664–670. doi: 10.1128/iai.48.3.664-670.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanapka J. A., Kleinberg I. Catabolism of arginine by the mixed bacteria in human salivary sediment under conditions of low and high glucose concentration. Arch Oral Biol. 1983;28(11):1007–1015. doi: 10.1016/0003-9969(83)90055-9. [DOI] [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Bender G. R., Murray D. R., Wong A. Arginine deiminase system and bacterial adaptation to acid environments. Appl Environ Microbiol. 1987 Jan;53(1):198–200. doi: 10.1128/aem.53.1.198-200.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Porterfield N., Matsumura P. Acid-base titration of streptococci and the physical states of intracellular ions. J Bacteriol. 1973 May;114(2):491–498. doi: 10.1128/jb.114.2.491-498.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Jensen M. E. Comparison of methods for monitoring changes in the pH of human dental plaque. J Dent Res. 1982 Oct;61(10):1117–1125. doi: 10.1177/00220345820610100201. [DOI] [PubMed] [Google Scholar]
- Sutton S. V., Marquis R. E. Membrane-associated and solubilized ATPases of Streptococcus mutans and Streptococcus sanguis. J Dent Res. 1987 Jun;66(6):1095–1098. doi: 10.1177/00220345870660060201. [DOI] [PubMed] [Google Scholar]
- de Jong M. H., van der Hoeven J. S., van OS J. H., Olijve J. H. Growth of oral Streptococcus species and Actinomyces viscosus in human saliva. Appl Environ Microbiol. 1984 May;47(5):901–904. doi: 10.1128/aem.47.5.901-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



