Abstract
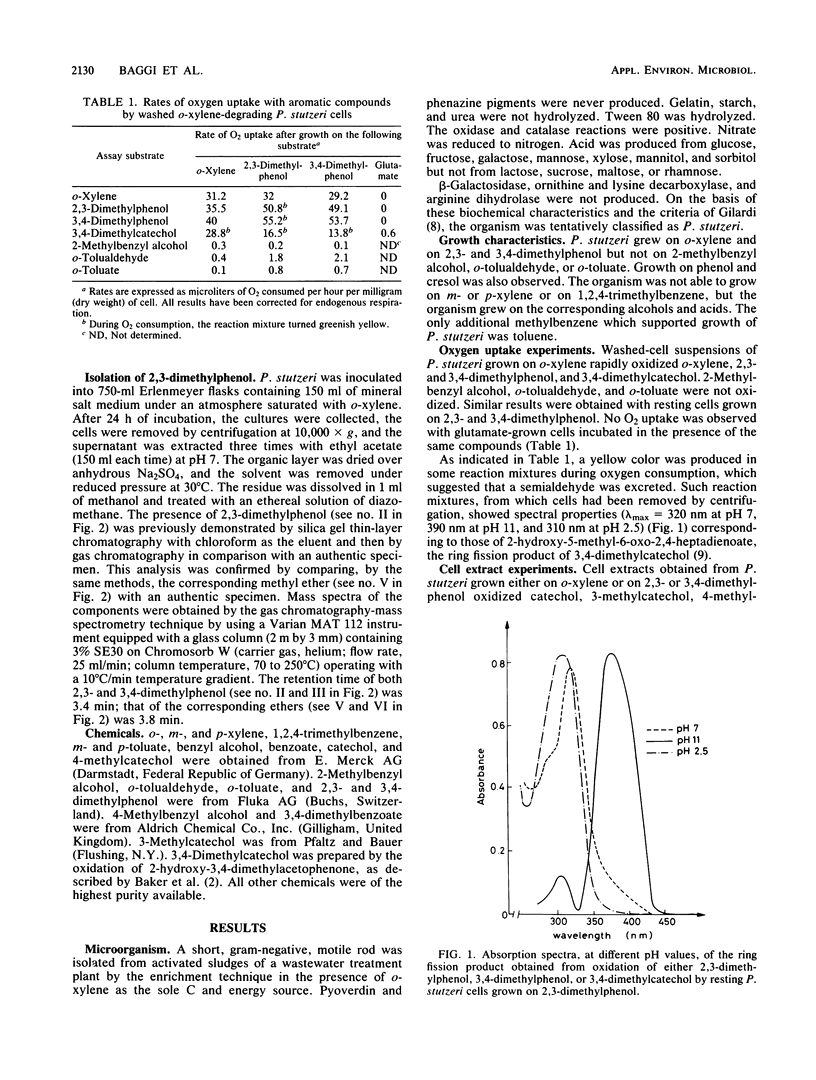
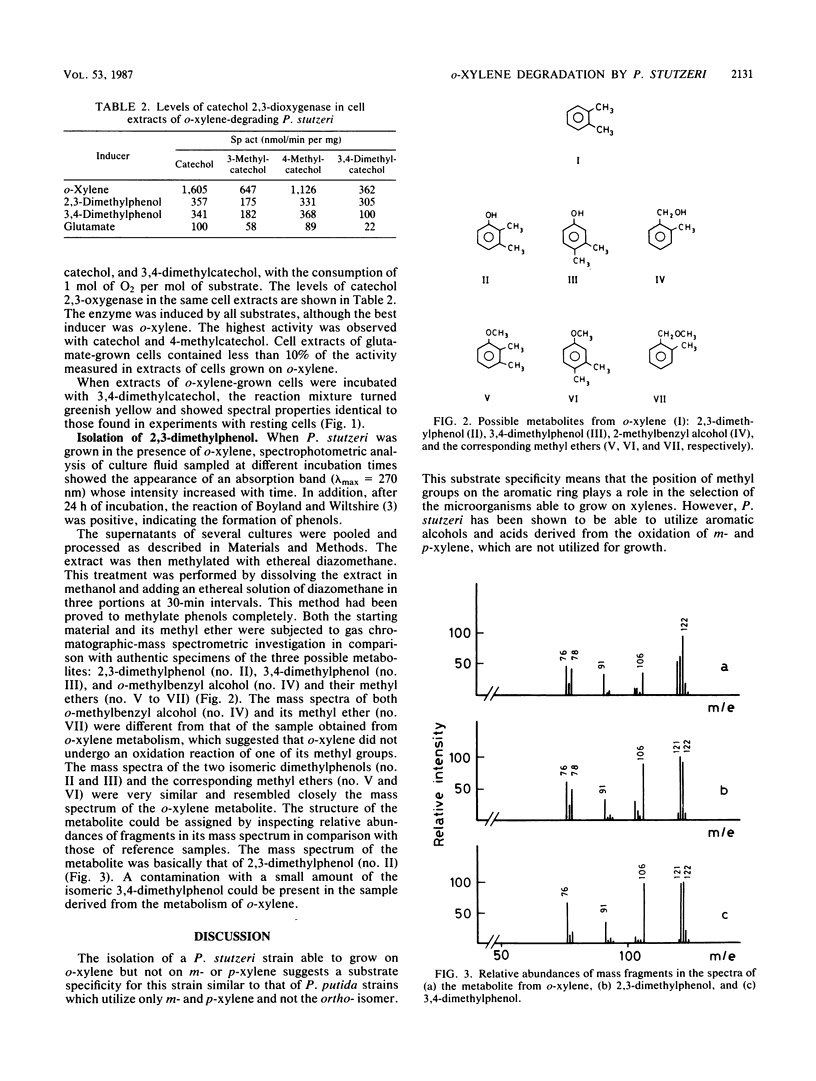
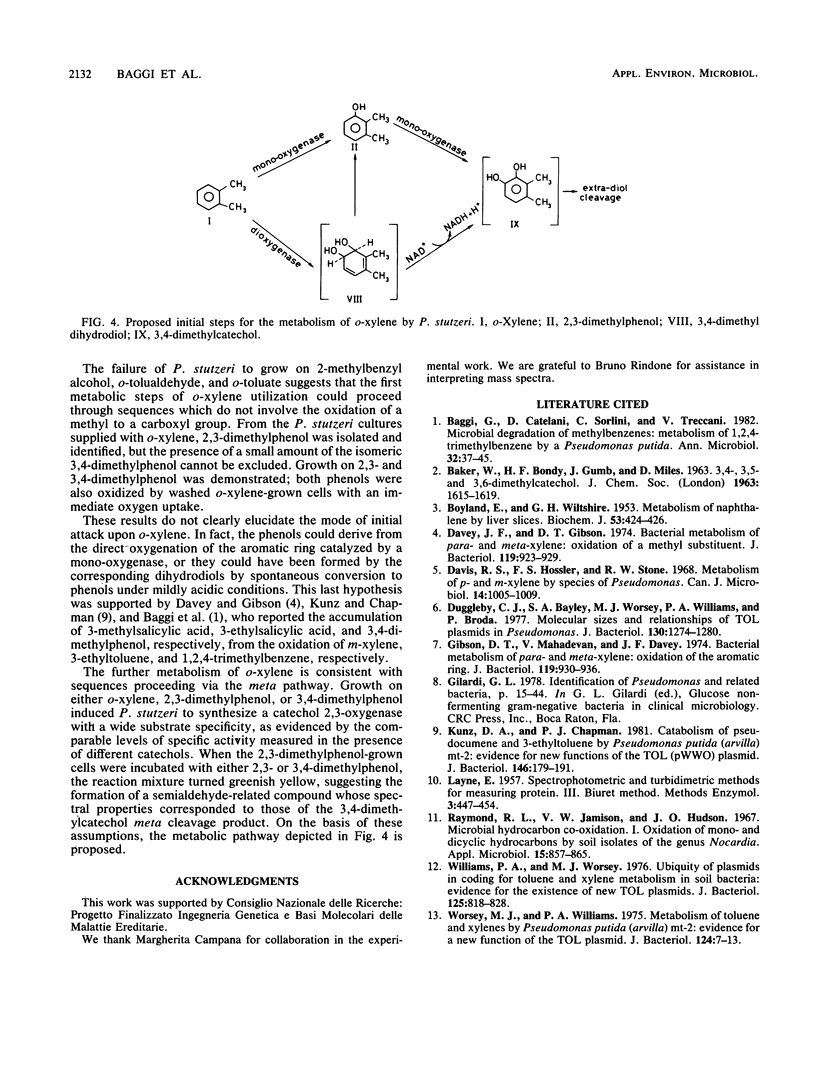
A Pseudomonas stutzeri strain capable of growing on o-xylene was isolated from enrichment cultures. The organism grew on 2,3- and 3,4-dimethylphenol but not on 2-methylbenzyl alcohol, o-tolualdehyde, or o-toluate. P. stutzeri was not able to utilize m-xylene, p-xylene, or 1,2,4-trimethylbenzene, but growth was observed in the presence of the corresponding alcohols and acids. From the Pseudomonas cultures supplied with o-xylene, 2,3-dimethylphenol was isolated and identified. When resting P. stutzeri cells were incubated with 2,3-dimethylphenol, the reaction mixture turned greenish yellow and showed spectral properties identical to those of the 3,4-dimethylcatechol meta ring fission product. Catechol 2,3-oxygenase was induced by growth on o-xylene or on 2,3- or 3,4-dimethylphenol. The suggested hypothesis is that the first metabolic steps of growth on o-xylene involve the direct oxygenation of the aromatic nucleus, followed by meta pathway reactions.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYLAND E., WILTSHIRE G. H. Metabolism of naphthalene by liver slices. Biochem J. 1953 Feb;53(3):424–426. doi: 10.1042/bj0530424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J. F., Gibson D. T. Bacterial metabolism of para- and meta-xylene: oxidation of a methyl substituent. J Bacteriol. 1974 Sep;119(3):923–929. doi: 10.1128/jb.119.3.923-929.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. S., Hossler F. E., Stone R. W. Metabolism of p- and m-xylene by species of Pseudomonas. Can J Microbiol. 1968 Sep;14(9):1005–1009. doi: 10.1139/m68-166. [DOI] [PubMed] [Google Scholar]
- Duggleby C. J., Bayley S. A., Worsey M. J., Williams P. A., Broda P. Molecular sizes and relationships of TOL plasmids in Pseudomonas. J Bacteriol. 1977 Jun;130(3):1274–1280. doi: 10.1128/jb.130.3.1274-1280.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Mahadevan V., Davey J. F. Bacterial metabolism of para- and meta-xylene: oxidation of the aromatic ring. J Bacteriol. 1974 Sep;119(3):930–936. doi: 10.1128/jb.119.3.930-936.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz D. A., Chapman P. J. Catabolism of pseudocumene and 3-ethyltoluene by Pseudomonas putida (arvilla) mt-2: evidence for new functions of the TOL (pWWO) plasmid. J Bacteriol. 1981 Apr;146(1):179–191. doi: 10.1128/jb.146.1.179-191.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond R. L., Jamison V. W., Hudson J. O. Microbial hydrocarbon co-oxidation. I. Oxidation of mono- and dicyclic hydrocarbons by soil isolates of the genus Nocardia. Appl Microbiol. 1967 Jul;15(4):857–865. doi: 10.1128/am.15.4.857-865.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. A., Worsey M. J. Ubiquity of plasmids in coding for toluene and xylene metabolism in soil bacteria: evidence for the existence of new TOL plasmids. J Bacteriol. 1976 Mar;125(3):818–828. doi: 10.1128/jb.125.3.818-828.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



