Abstract
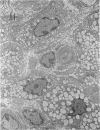
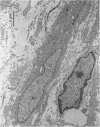
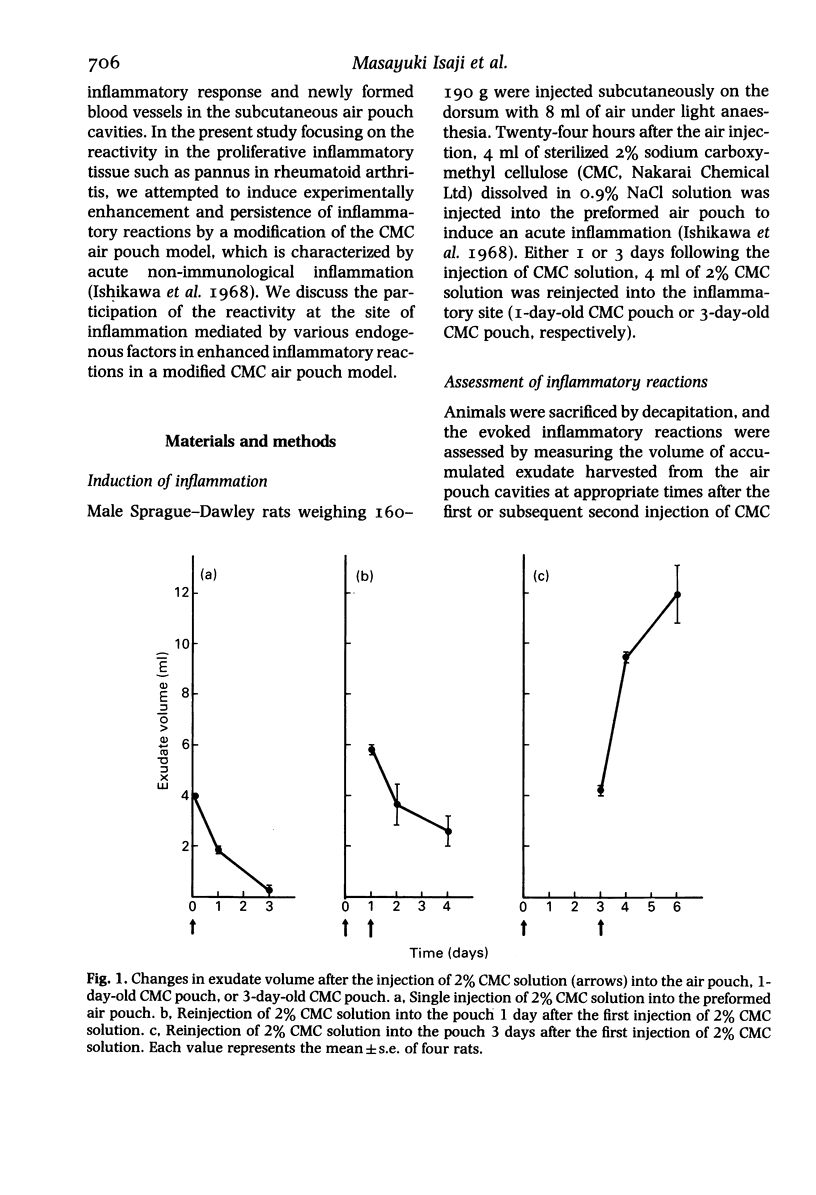
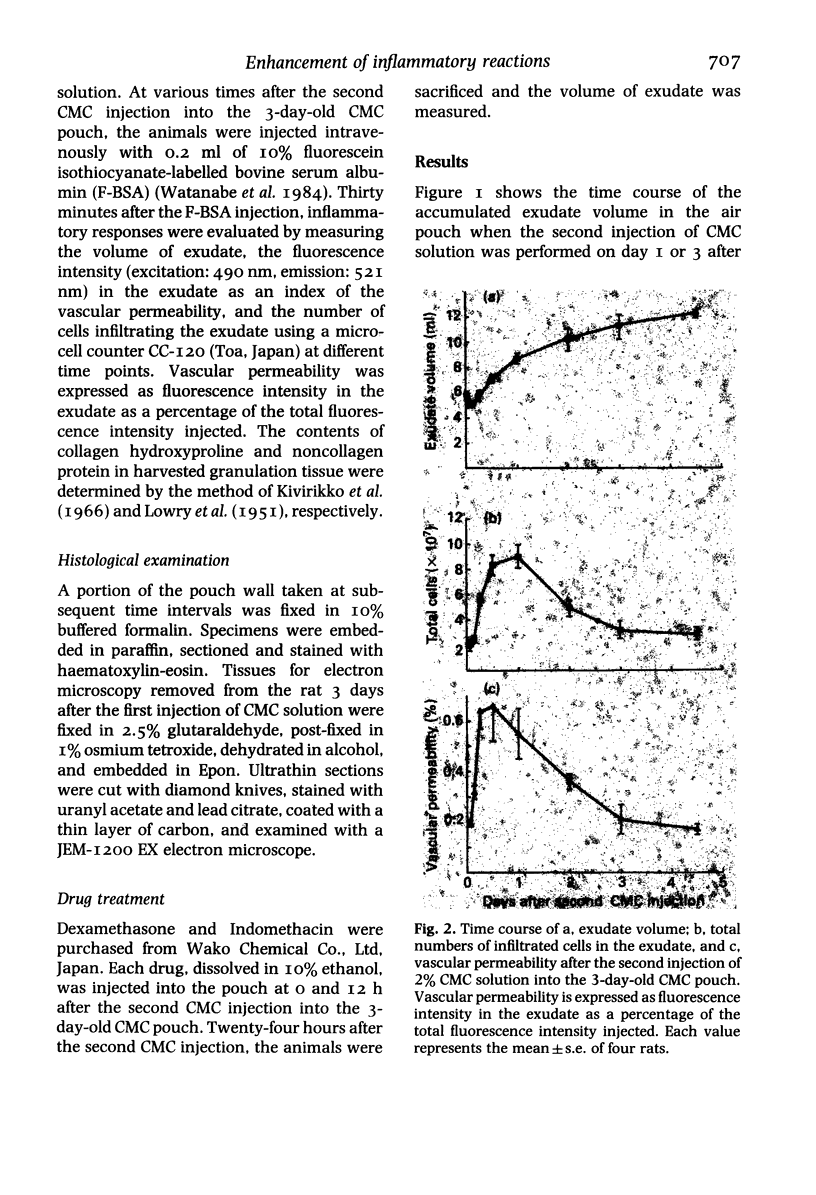
In a carboxymethyl cellulose (CMC) air pouch inflammation model, accumulation of exudate decreased at a relatively rapid rate and almost disappeared 3 days after a 2% CMC injection into the preformed air pouch. After a second injection of 2% CMC solution into the 1-day-old CMC pouch on the day following the first CMC injection, the decrease in rate of exudate was similar to the change seen after the first CMC injection. In another group of rats, 3 days after the first CMC injection when inflammation had subsided, a second injection of 2% CMC solution into the 3-day-old CMC pouch resulted in a marked increase of exudate accumulation, inflammatory cell infiltration and vascular permeability. Histologically, large numbers of macrophages accumulated in the 3-day-old CMC pouch and fibroblast proliferation and newly formed blood vessels were also visible. The enhanced exudative reaction was significantly inhibited by dexamethasone but not by indomethacin. These results indicate that the enhanced inflammatory reactions appear to be closely correlated with the increase of reactivity at the site of inflammation and the exudative reaction was not mediated by cyclo-oxygenase products.
Full text
PDF

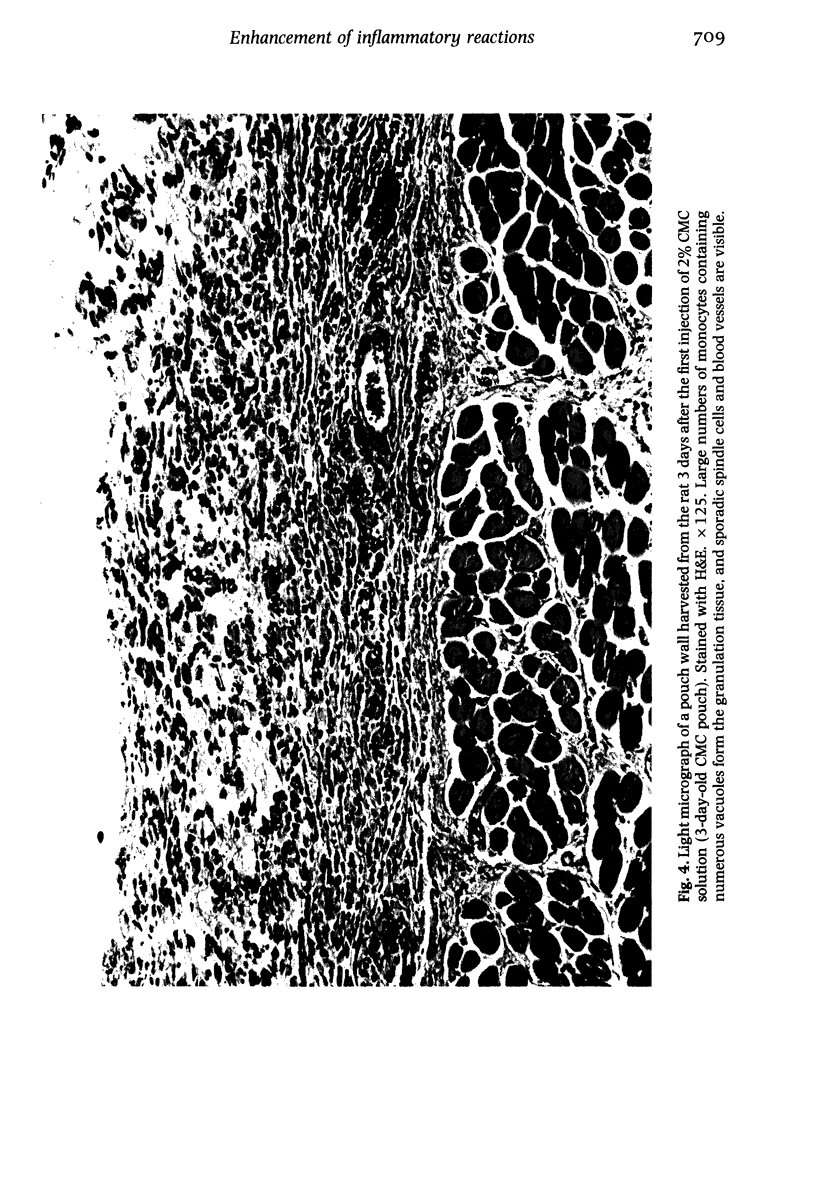
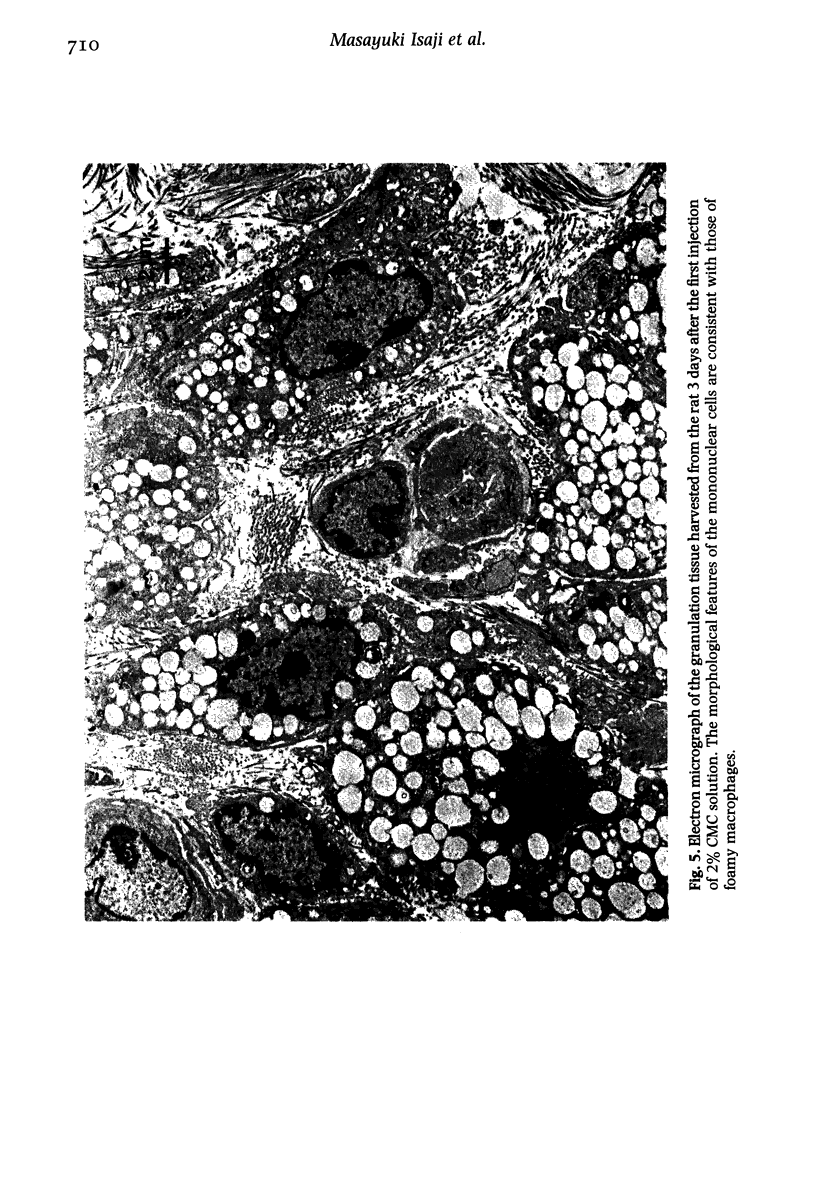
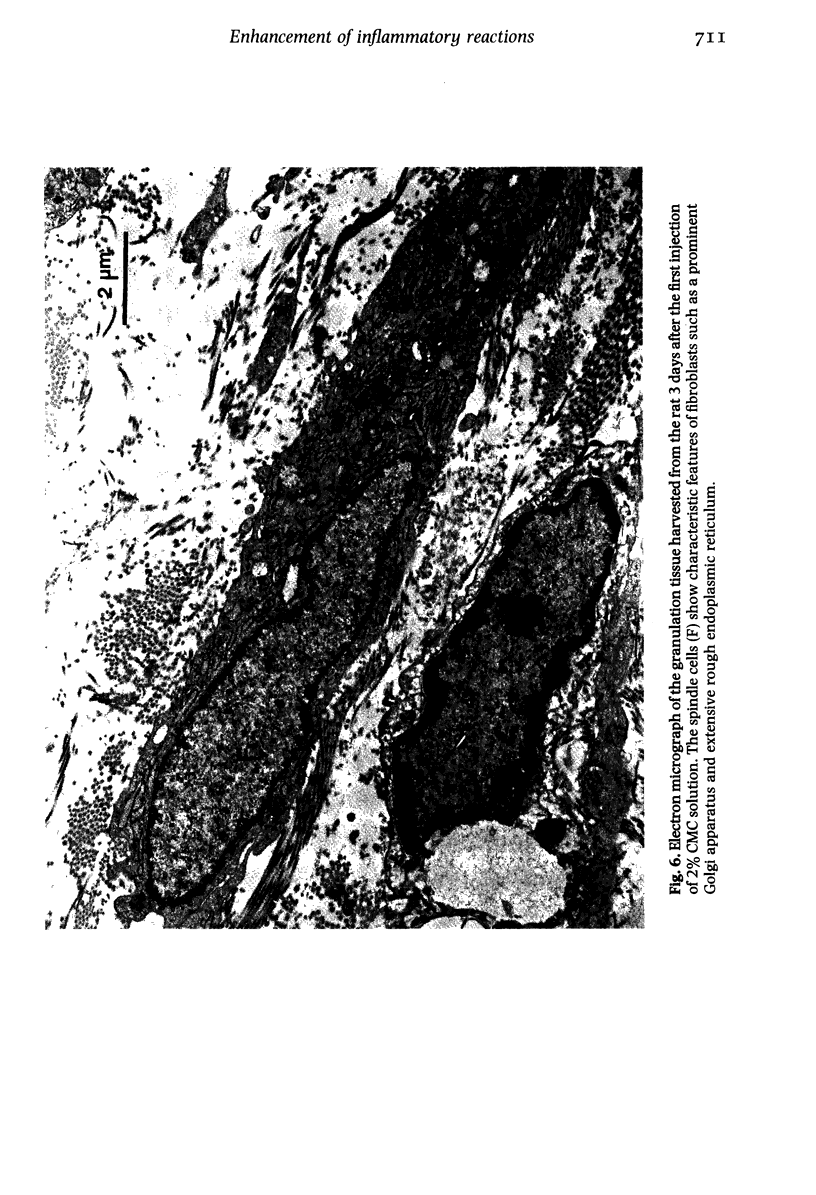





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasubramaniam G. S., Hurley J. V. The structure and reactions of the microcirculation in a subcutaneous air pouch in the rat. J Pathol. 1987 Feb;151(2):139–146. doi: 10.1002/path.1711510207. [DOI] [PubMed] [Google Scholar]
- Bertez C., Miquel M., Coquelet C., Sincholle D., Bonne C. Dual inhibition of cyclooxygenase and lipoxygenase by 2-acetylthiophene 2-thiazolylhydrazone (CBS-1108) and effect on leukocyte migration in vivo. Biochem Pharmacol. 1984 Jun 1;33(11):1757–1762. doi: 10.1016/0006-2952(84)90346-0. [DOI] [PubMed] [Google Scholar]
- Bird J., Sheng Y. J., Florentin I., Giroud J. P. Release of interleukin I and low-molecular-weight lymphocyte-activating factors by rat peritoneal macrophages and its enhancement by acute non-specific inflammatory processes. Br J Exp Pathol. 1985 Jun;66(3):271–277. [PMC free article] [PubMed] [Google Scholar]
- Cater D. B., Wallington T. B. Inflammatory changes in newly formed vessels of carrageenin-induced granulomas after systemic 5-hydroxytryptamine, bradykinin, kallikrein, or lysolecithin. Br J Exp Pathol. 1968 Feb;49(1):74–80. [PMC free article] [PubMed] [Google Scholar]
- Chung K. F. Role of inflammation in the hyperreactivity of the airways in asthma. Thorax. 1986 Sep;41(9):657–662. doi: 10.1136/thx.41.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C., Sedgwick A. D., Willoughby D. A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981 Jun;134(2):147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Florentin I., Bird J., Le Garrec Y., Chung V., Giroud J. P. Modifications of host defence mechanisms by an acute non-immunological inflammatory reaction. Br J Exp Pathol. 1985 Jun;66(3):257–270. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Mori Y., Tsurufuji S. The characteristic feature of glucocorticoids after local application with reference to leucocyte migration and protein exudation. Eur J Pharmacol. 1969 Aug;7(2):201–205. doi: 10.1016/0014-2999(69)90011-9. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Niinobe S., Tsurufuji S. [Studies on the mode of action of anti-inflammatory agents. I. Quantitative analysis of anti-inflammatory effects by carboxymethyl cellulose pouch method]. Yakugaku Zasshi. 1968 Nov;88(11):1472–1477. doi: 10.1248/yakushi1947.88.11_1472. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- Nadel J. A. Inflammation and asthma. J Allergy Clin Immunol. 1984 May;73(5 Pt 2):651–653. doi: 10.1016/0091-6749(84)90299-9. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Sedgwick A. D., Sin Y. M., Edwards J. C., Willoughby D. A. Increased inflammatory reactivity in newly formed lining tissue. J Pathol. 1983 Dec;141(4):483–495. doi: 10.1002/path.1711410406. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Buckingham R. B., Prince R. K., Rodnan G. P. Products of activated mononuclear cells modulate accumulation of collagen by normal dermal and scleroderma fibroblasts in culture. J Lab Clin Med. 1984 Sep;104(3):355–369. [PubMed] [Google Scholar]