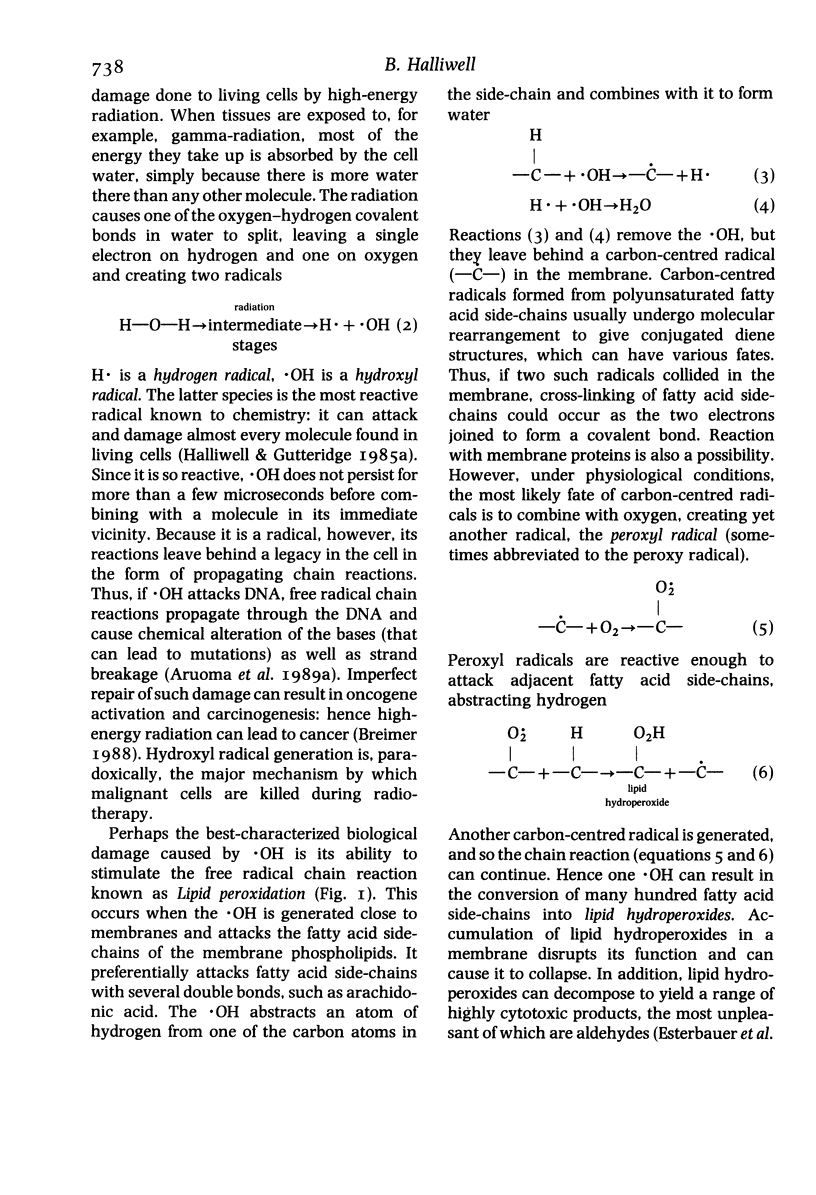
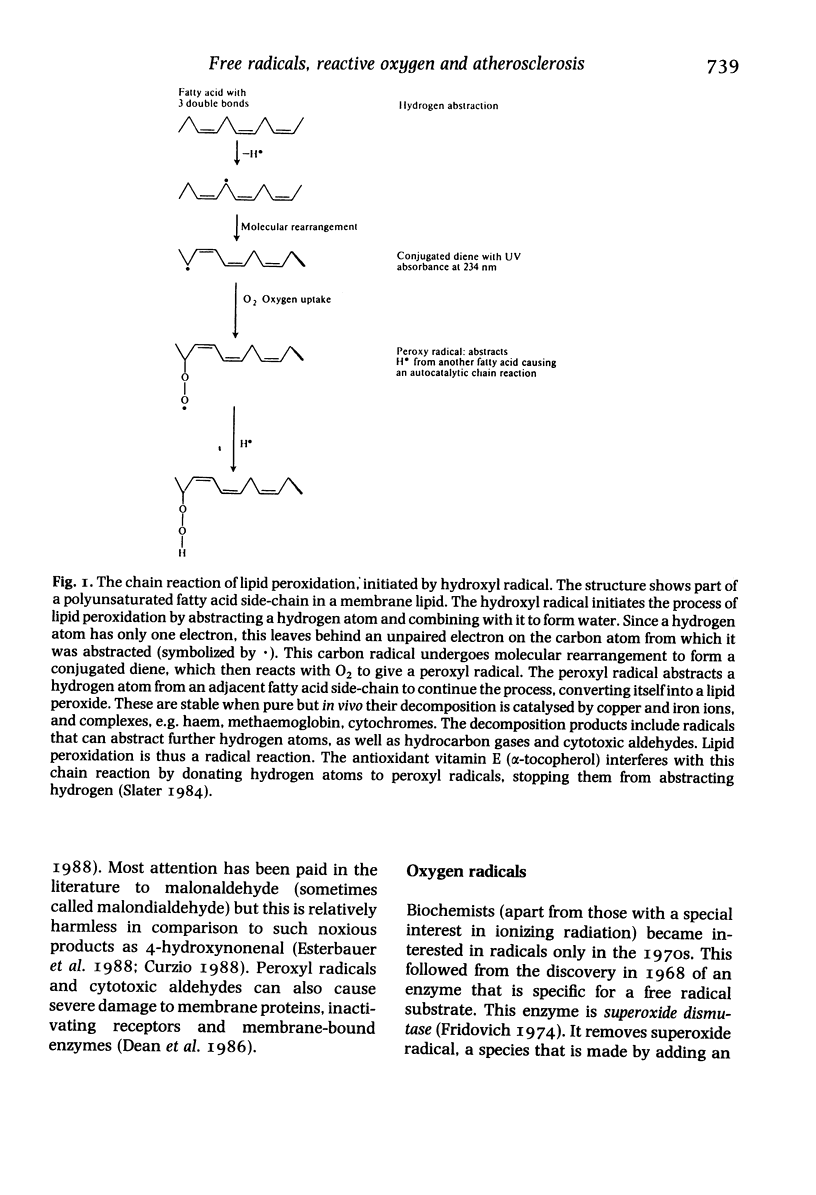
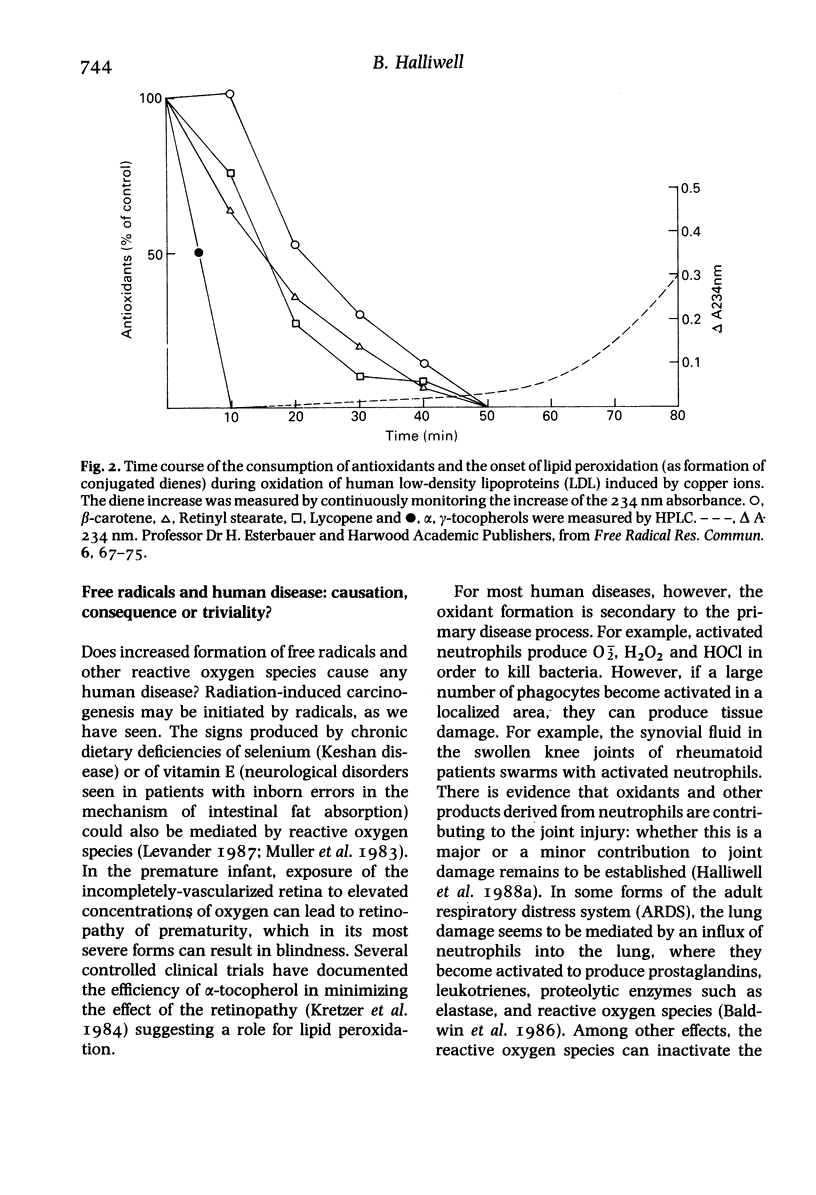
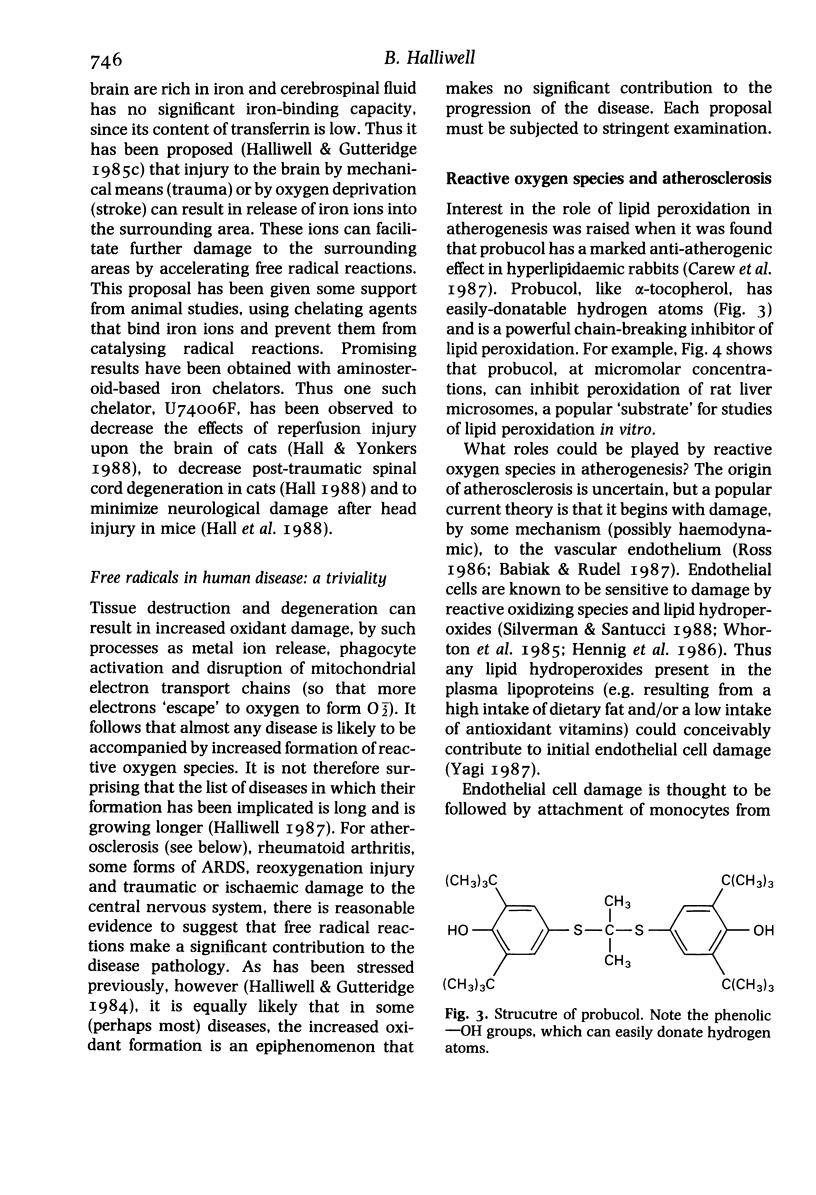
Full text
PDF





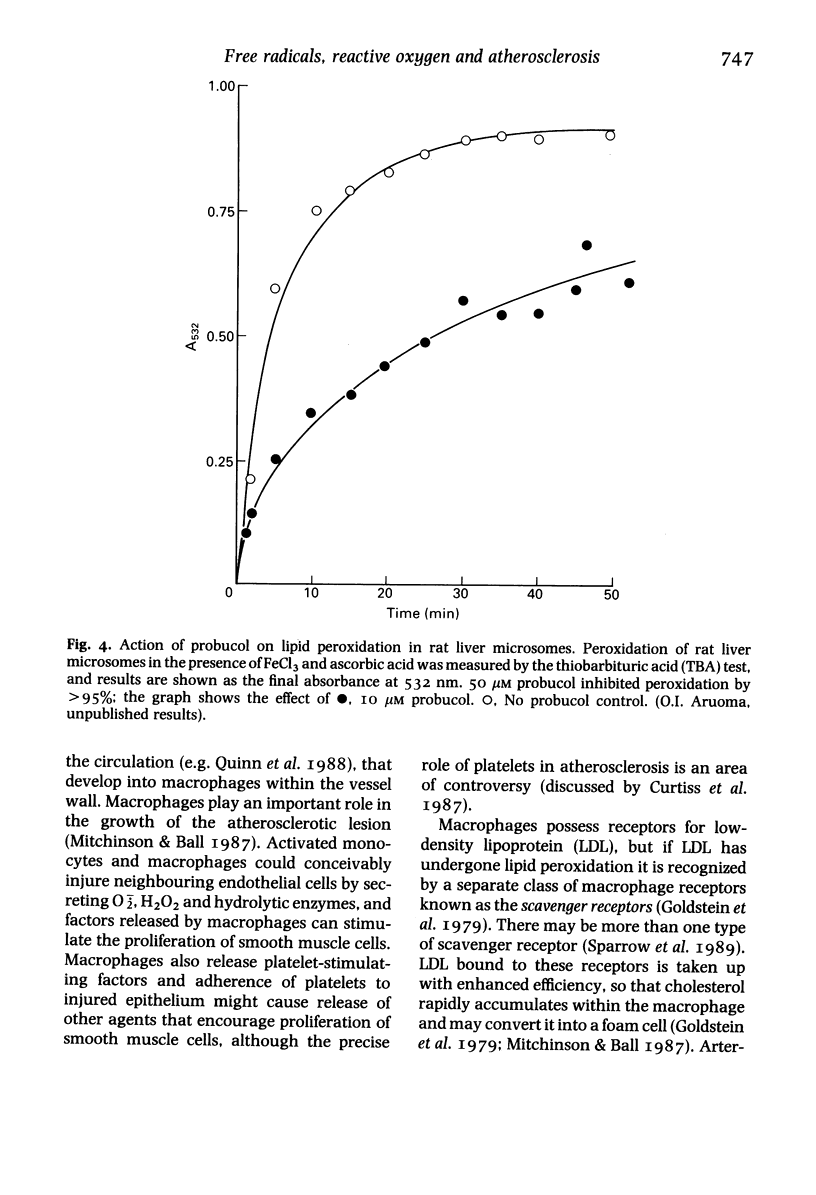










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Bomford A., Polson R. J., Halliwell B. Nontransferrin-bound iron in plasma from hemochromatosis patients: effect of phlebotomy therapy. Blood. 1988 Oct;72(4):1416–1419. [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Laughton M. J., Quinlan G. J., Gutteridge J. M. The mechanism of initiation of lipid peroxidation. Evidence against a requirement for an iron(II)-iron(III) complex. Biochem J. 1989 Mar 1;258(2):617–620. doi: 10.1042/bj2580617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987 Jan 1;241(1):273–278. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball R. Y., Carpenter K. L., Mitchinson M. J. What is the significance of ceroid in human atherosclerosis? Arch Pathol Lab Med. 1987 Dec;111(12):1134–1140. [PubMed] [Google Scholar]
- Bernheimer A. W., Robinson W. G., Linder R., Mullins D., Yip Y. K., Cooper N. S., Seidman I., Uwajima T. Toxicity of enzymically-oxidized low-density lipoprotein. Biochem Biophys Res Commun. 1987 Oct 14;148(1):260–266. doi: 10.1016/0006-291x(87)91104-1. [DOI] [PubMed] [Google Scholar]
- Bird R. P., Hung S. S., Hadley M., Draper H. H. Determination of malonaldehyde in biological materials by high-pressure liquid chromatography. Anal Biochem. 1983 Jan;128(1):240–244. doi: 10.1016/0003-2697(83)90371-8. [DOI] [PubMed] [Google Scholar]
- Bolli R. Oxygen-derived free radicals and postischemic myocardial dysfunction ("stunned myocardium"). J Am Coll Cardiol. 1988 Jul;12(1):239–249. doi: 10.1016/0735-1097(88)90381-6. [DOI] [PubMed] [Google Scholar]
- Breimer L. H. Ionizing radiation-induced mutagenesis. Br J Cancer. 1988 Jan;57(1):6–18. doi: 10.1038/bjc.1988.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart M. K., McNally A. K., Morel D. W., Chisolm G. M., 3rd Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein and conversion of low-density lipoprotein to a cytotoxin. J Immunol. 1989 Mar 15;142(6):1963–1969. [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Chronic granulomatous disease. Adv Hum Genet. 1987;16:229–297. doi: 10.1007/978-1-4757-0620-8_4. [DOI] [PubMed] [Google Scholar]
- Curtiss L. K., Black A. S., Takagi Y., Plow E. F. New mechanism for foam cell generation in atherosclerotic lesions. J Clin Invest. 1987 Aug;80(2):367–373. doi: 10.1172/JCI113081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzio M. Interaction between neutrophils and 4-hydroxyalkenals and consequences on neutrophil motility. Free Radic Res Commun. 1988;5(2):55–66. doi: 10.3109/10715768809066912. [DOI] [PubMed] [Google Scholar]
- Czapski G., Goldstein S., Meyerstein D. What is unique about superoxide toxicity as compared to other biological reductants? A hypothesis. Free Radic Res Commun. 1988;4(4):231–236. doi: 10.3109/10715768809055147. [DOI] [PubMed] [Google Scholar]
- Daniels V. Oxidative damage and the preservation of organic artefacts. Free Radic Res Commun. 1989;5(4-5):213–220. doi: 10.3109/10715768909074703. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Thomas S. M., Garner A. Free-radical-mediated fragmentation of monoamine oxidase in the mitochondrial membrane. Roles for lipid radicals. Biochem J. 1986 Dec 1;240(2):489–494. doi: 10.1042/bj2400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormandy T. L., Wickens D. G. The experimental and clinical pathology of diene conjugation. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):353–364. doi: 10.1016/0009-3084(87)90072-7. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Striegl G., Puhl H., Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6(1):67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- Fairbank J., Ridgway L., Griffin J., Wickens D., Singer A., Dormandy T. L. Octadeca-9-11-dienoic acid in diagnosis of cervical intraepithelial neoplasia. Lancet. 1988 Aug 6;2(8606):329–330. doi: 10.1016/s0140-6736(88)92375-6. [DOI] [PubMed] [Google Scholar]
- Farr S. B., D'Ari R., Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Gey K. F., Brubacher G. B., Stähelin H. B. Plasma levels of antioxidant vitamins in relation to ischemic heart disease and cancer. Am J Clin Nutr. 1987 May;45(5 Suppl):1368–1377. doi: 10.1093/ajcn/45.5.1368. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. Guanine nucleotide-binding regulatory proteins and dual control of adenylate cyclase. J Clin Invest. 1984 Jan;73(1):1–4. doi: 10.1172/JCI111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootveld M., Bell J. D., Halliwell B., Aruoma O. I., Bomford A., Sadler P. J. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989 Mar 15;264(8):4417–4422. [PubMed] [Google Scholar]
- Grootveld M., Jain R. Recent advances in the development of a diagnostic test for irradiated foodstuffs. Free Radic Res Commun. 1989;6(5):271–292. doi: 10.3109/10715768909055153. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Copper-phenanthroline-induced site-specific oxygen-radical damage to DNA. Detection of loosely bound trace copper in biological fluids. Biochem J. 1984 Mar 15;218(3):983–985. doi: 10.1042/bj2180983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Richmond R., Halliwell B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J. 1979 Nov 15;184(2):469–472. doi: 10.1042/bj1840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Thiobarbituric acid-reactivity following iron-dependent free-radical damage to amino acids and carbohydrates. FEBS Lett. 1981 Jun 15;128(2):343–346. doi: 10.1016/0014-5793(81)80113-5. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Tickner T. R. The characterisation of thiobarbituric acid reactivity in human plasma and urine. Anal Biochem. 1978 Nov;91(1):250–257. doi: 10.1016/0003-2697(78)90838-2. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Tickner T. R. The thiobarbituric acid-reactivity of bile pigments. Biochem Med. 1978 Feb;19(1):127–132. doi: 10.1016/0006-2944(78)90013-3. [DOI] [PubMed] [Google Scholar]
- Hall E. D. Effects of the 21-aminosteroid U74006F on posttraumatic spinal cord ischemia in cats. J Neurosurg. 1988 Mar;68(3):462–465. doi: 10.3171/jns.1988.68.3.0462. [DOI] [PubMed] [Google Scholar]
- Hall E. D., Yonkers P. A. Attenuation of postischemic cerebral hypoperfusion by the 21-aminosteroid U74006F. Stroke. 1988 Mar;19(3):340–344. doi: 10.1161/01.str.19.3.340. [DOI] [PubMed] [Google Scholar]
- Hall E. D., Yonkers P. A., McCall J. M., Braughler J. M. Effects of the 21-aminosteroid U74006F on experimental head injury in mice. J Neurosurg. 1988 Mar;68(3):456–461. doi: 10.3171/jns.1988.68.3.0456. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Albumin--an important extracellular antioxidant? Biochem Pharmacol. 1988 Feb 15;37(4):569–571. doi: 10.1016/0006-2952(88)90126-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Aruoma O. I., Mufti G., Bomford A. Bleomycin-detectable iron in serum from leukaemic patients before and after chemotherapy. Therapeutic implications for treatment with oxidant-generating drugs. FEBS Lett. 1988 Dec 5;241(1-2):202–204. doi: 10.1016/0014-5793(88)81061-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984 Jun 23;1(8391):1396–1397. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Hoult J. R., Blake D. R. Oxidants, inflammation, and anti-inflammatory drugs. FASEB J. 1988 Oct;2(13):2867–2873. doi: 10.1096/fasebj.2.13.2844616. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W., Baker L., Rosen H., Chait A. Superoxide-mediated modification of low density lipoprotein by arterial smooth muscle cells. J Clin Invest. 1986 Mar;77(3):757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B., Enoch C., Chow C. K. Linoleic acid hydroperoxide increases the transfer of albumin across cultured endothelial monolayers. Arch Biochem Biophys. 1986 Jul;248(1):353–357. doi: 10.1016/0003-9861(86)90431-5. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H. A. Oxygen, oxidases, and the essential trace metals. Philos Trans R Soc Lond B Biol Sci. 1981 Aug 14;294(1071):119–128. doi: 10.1098/rstb.1981.0093. [DOI] [PubMed] [Google Scholar]
- Holland J. A., Pritchard K. A., Rogers N. J., Stemerman M. B. Perturbation of cultured human endothelial cells by atherogenic levels of low density lipoprotein. Am J Pathol. 1988 Sep;132(3):474–478. [PMC free article] [PubMed] [Google Scholar]
- Ingold K. U., Burton G. W., Foster D. O., Zuker M., Hughes L., Lacelle S., Lusztyk E., Slaby M. A new vitamin E analogue more active than alpha-tocopherol in the rat curative myopathy bioassay. FEBS Lett. 1986 Sep 1;205(1):117–120. doi: 10.1016/0014-5793(86)80877-8. [DOI] [PubMed] [Google Scholar]
- Jürgens G., Hoff H. F., Chisolm G. M., 3rd, Esterbauer H. Modification of human serum low density lipoprotein by oxidation--characterization and pathophysiological implications. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):315–336. doi: 10.1016/0009-3084(87)90070-3. [DOI] [PubMed] [Google Scholar]
- Kehrer J. P. Concepts related to the study of reactive oxygen and cardiac reperfusion injury. Free Radic Res Commun. 1989;5(6):305–314. doi: 10.3109/10715768909073412. [DOI] [PubMed] [Google Scholar]
- Kosugi H., Kato T., Kikugawa K. Formation of yellow, orange, and red pigments in the reaction of alk-2-enals with 2-thiobarbituric acid. Anal Biochem. 1987 Sep;165(2):456–464. doi: 10.1016/0003-2697(87)90296-x. [DOI] [PubMed] [Google Scholar]
- Kretzer F. L., Mehta R. S., Johnson A. T., Hunter D. G., Brown E. S., Hittner H. M. Vitamin E protects against retinopathy of prematurity through action on spindle cells. 1984 Jun 28-Jul 4Nature. 309(5971):793–795. doi: 10.1038/309793a0. [DOI] [PubMed] [Google Scholar]
- Levander O. A. A global view of human selenium nutrition. Annu Rev Nutr. 1987;7:227–250. doi: 10.1146/annurev.nu.07.070187.001303. [DOI] [PubMed] [Google Scholar]
- Marcillat O., Zhang Y., Lin S. W., Davies K. J. Mitochondria contain a proteolytic system which can recognize and degrade oxidatively-denatured proteins. Biochem J. 1988 Sep 15;254(3):677–683. doi: 10.1042/bj2540677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Role of toxic effects of oxygen in reperfusion damage. J Mol Cell Cardiol. 1988 Mar;20 (Suppl 2):23–30. doi: 10.1016/0022-2828(88)90329-x. [DOI] [PubMed] [Google Scholar]
- Marshall P. J., Warso M. A., Lands W. E. Selective microdetermination of lipid hydroperoxides. Anal Biochem. 1985 Feb 15;145(1):192–199. doi: 10.1016/0003-2697(85)90347-1. [DOI] [PubMed] [Google Scholar]
- McCay P. B. Vitamin E: interactions with free radicals and ascorbate. Annu Rev Nutr. 1985;5:323–340. doi: 10.1146/annurev.nu.05.070185.001543. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McLaren G. D., Muir W. A., Kellermeyer R. W. Iron overload disorders: natural history, pathogenesis, diagnosis, and therapy. Crit Rev Clin Lab Sci. 1983;19(3):205–266. doi: 10.3109/10408368309165764. [DOI] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. The role of iron in the initiation of lipid peroxidation. Chem Phys Lipids. 1987 Jul-Sep;44(2-4):191–208. doi: 10.1016/0009-3084(87)90050-8. [DOI] [PubMed] [Google Scholar]
- Mitchinson M. J., Ball R. Y. Macrophages and atherogenesis. Lancet. 1987 Jul 18;2(8551):146–148. doi: 10.1016/s0140-6736(87)92341-5. [DOI] [PubMed] [Google Scholar]
- Muller D. P., Lloyd J. K., Wolff O. H. Vitamin E and neurological function. Lancet. 1983 Jan 29;1(8318):225–228. doi: 10.1016/s0140-6736(83)92598-9. [DOI] [PubMed] [Google Scholar]
- Pacht E. R., Kaseki H., Mohammed J. R., Cornwell D. G., Davis W. B. Deficiency of vitamin E in the alveolar fluid of cigarette smokers. Influence on alveolar macrophage cytotoxicity. J Clin Invest. 1986 Mar;77(3):789–796. doi: 10.1172/JCI112376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Wieland E., Steinberg D. A role for endothelial cell lipoxygenase in the oxidative modification of low density lipoprotein. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1046–1050. doi: 10.1073/pnas.86.3.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Sanan S., Sharma G., Malhotra R., Sanan D. P., Jain P., Vadhera P. Protection by desferrioxamine against histopathological changes of the liver in the post-oligaemic phase of clinical haemorrhagic shock in dogs: correlation with improved survival rate and recovery. Free Radic Res Commun. 1989;6(1):29–38. doi: 10.3109/10715768909073425. [DOI] [PubMed] [Google Scholar]
- Scott G. Potential toxicological problems associated with antioxidants in plastics and rubber consumables. Free Radic Res Commun. 1988;5(3):141–147. doi: 10.3109/10715768809066923. [DOI] [PubMed] [Google Scholar]
- Sevanian A., Peterson A. R. The cytotoxic and mutagenic properties of cholesterol oxidation products. Food Chem Toxicol. 1986 Oct-Nov;24(10-11):1103–1110. doi: 10.1016/0278-6915(86)90295-4. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kondo K., Hayaishi O. Role of prostaglandin endoperoxides in the serum thiobarbituric acid reaction. Arch Biochem Biophys. 1981 Feb;206(2):271–276. doi: 10.1016/0003-9861(81)90091-6. [DOI] [PubMed] [Google Scholar]
- Silverman D. J., Santucci L. A. Potential for free radical-induced lipid peroxidation as a cause of endothelial cell injury in Rocky Mountain spotted fever. Infect Immun. 1988 Dec;56(12):3110–3115. doi: 10.1128/iai.56.12.3110-3115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon J. M., Vane J. R. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow C. P., Parthasarathy S., Steinberg D. A macrophage receptor that recognizes oxidized low density lipoprotein but not acetylated low density lipoprotein. J Biol Chem. 1989 Feb 15;264(5):2599–2604. [PubMed] [Google Scholar]
- Steinbrecher U. P. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987 Mar 15;262(8):3603–3608. [PubMed] [Google Scholar]
- Swartz G. M., Jr, Gentry M. K., Amende L. M., Blanchette-Mackie E. J., Alving C. R. Antibodies to cholesterol. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1902–1906. doi: 10.1073/pnas.85.6.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S., Smith M. T. Measurement of the diene conjugated form of linoleic acid in plasma by high performance liquid chromatography: a questionable non-invasive assay of free radical activity? Chem Biol Interact. 1985 Nov;55(3):357–366. doi: 10.1016/s0009-2797(85)80142-3. [DOI] [PubMed] [Google Scholar]
- Wallenburg H. C., Dekker G. A., Makovitz J. W., Rotmans P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet. 1986 Jan 4;1(8471):1–3. doi: 10.1016/s0140-6736(86)91891-x. [DOI] [PubMed] [Google Scholar]
- Warso M. A., Lands W. E. Lipid peroxidation in relation to prostacyclin and thromboxane physiology and pathophysiology. Br Med Bull. 1983 Jul;39(3):277–280. doi: 10.1093/oxfordjournals.bmb.a071833. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Whorton A. R., Montgomery M. E., Kent R. S. Effect of hydrogen peroxide on prostaglandin production and cellular integrity in cultured porcine aortic endothelial cells. J Clin Invest. 1985 Jul;76(1):295–302. doi: 10.1172/JCI111960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokode M., Kita T., Kikawa Y., Ogorochi T., Narumiya S., Kawai C. Stimulated arachidonate metabolism during foam cell transformation of mouse peritoneal macrophages with oxidized low density lipoprotein. J Clin Invest. 1988 Mar;81(3):720–729. doi: 10.1172/JCI113377. [DOI] [PMC free article] [PubMed] [Google Scholar]


