Abstract
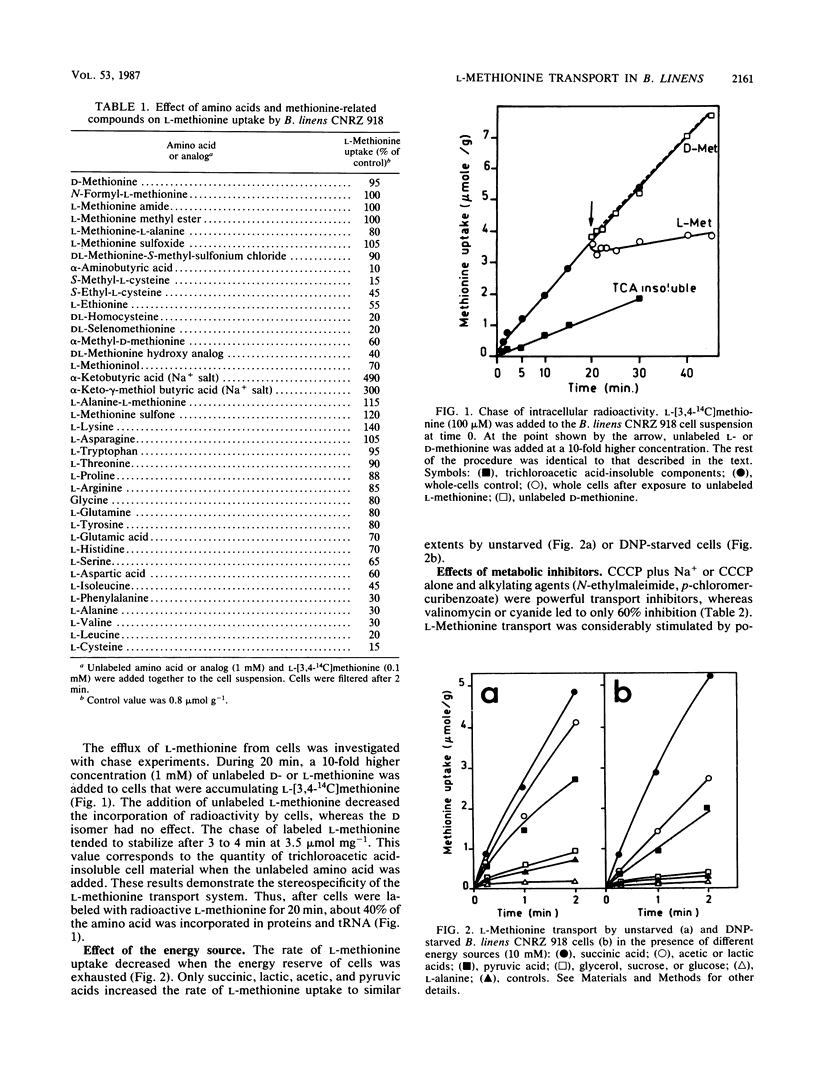
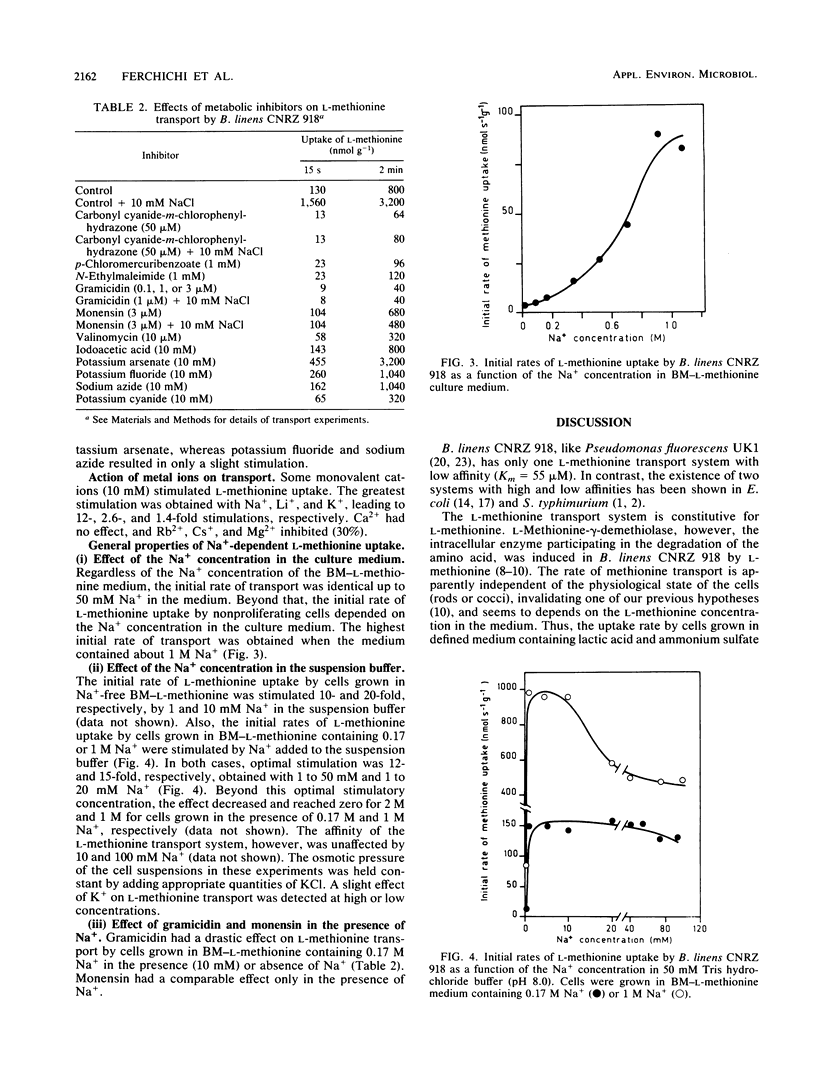
The transport of l-methionine by the gram-positive species Brevibacterium linens CNRZ 918 is described. The one transport system (Km = 55 μM) found is constitutive for l-methionine, stereospecific, and pH and temperature dependent. Entry of l-methionine into cells is controlled by the internal methionine pool. Competition studies indicate that l-methionine and α-aminobutyric acid share a common carrier for their transport. Neither methionine derivatives substituted on the amino or carboxyl groups nor d-methionine was an inhibitor, whereas powerful inhibition was shown by l-cysteine, s-methyl-l-cysteine, dl-selenomethionine and dl-homocysteine. Sodium plays important and varied roles in l-methionine transport by B. linens CNRZ 918: (i) it stimulates transport without affecting the Km, (ii) it increases the specific activity (on a biomass basis) of the l-methionine transport system when present with methionine in the medium, suggesting a coinduction mechanism. l-Methionine transport requires an exogenous energy source, which may be succinic, lactic, acetic, or pyruvic acid but not glucose or sucrose. The fact that l-methionine transport was stimulated by potassium arsenate and to a lesser extent by potassium fluoride suggests that high-energy phosphorylated intermediates are not involved in the process. Monensin eliminates stimulation by sodium. Gramicidin and carbonyl cyanide-m-chlorophenylhydrazone act in the presence or absence of Na+. N-Ethylmaleimide, p-chloromercurobenzoate, valinomycin, sodium azide, and potassium cyanide have no or only a partial inhibitory effect. These results tend to indicate that the proton motive force reinforced by the Na+ gradient is involved in the mechanism of energy coupling of l-methionine transport by B. linens CNRZ 918. Thus, this transport is partially similar to the well-described systems in gram-negative bacteria, except for the role of sodium, which is very effective in B. linens, a species adapted to the high sodium levels of its niche.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayling P. D., Bridgeland E. S. Methionine transport in wild-type and transport-defective mutants of Salmonella typhimurium. J Gen Microbiol. 1972 Nov;73(1):127–141. doi: 10.1099/00221287-73-1-127. [DOI] [PubMed] [Google Scholar]
- Ayling P. D., Mojica-a T., Klopotowski T. Methionine transport in Salmonella typhimurium: evidence for at least one low-affinity transport system. J Gen Microbiol. 1979 Oct;114(2):227–246. doi: 10.1099/00221287-114-2-227. [DOI] [PubMed] [Google Scholar]
- Boyaval P., Moreira E., Desmazeaud M. J. Electrochemical proton gradient of Brevibacterium linens and its relationship to phenylalanine transport. Ann Microbiol (Paris) 1984 Jul-Aug;135B(1):91–99. doi: 10.1016/s0769-2609(84)80046-0. [DOI] [PubMed] [Google Scholar]
- Boyaval P., Moreira E., Desmazeaud M. J. Transport of aromatic amino acids by Brevibacterium linens. J Bacteriol. 1983 Sep;155(3):1123–1129. doi: 10.1128/jb.155.3.1123-1129.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. Utilization of d-Methionine by Escherichia coli. J Bacteriol. 1966 Aug;92(2):328–332. doi: 10.1128/jb.92.2.328-332.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferchichi M., Hemme D., Bouillanne C. Influence of Oxygen and pH on Methanethiol Production from l-Methionine by Brevibacterium linens CNRZ 918. Appl Environ Microbiol. 1986 Apr;51(4):725–729. doi: 10.1128/aem.51.4.725-729.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferchichi M., Hemme D., Nardi M., Pamboukdjian N. Production of methanethiol from methionine by Brevibacterium linens CNRZ 918. J Gen Microbiol. 1985 Apr;131(4):715–723. doi: 10.1099/00221287-131-4-715. [DOI] [PubMed] [Google Scholar]
- Kadner R. J. Regulation of methionine transport activity in Escherichia coli. J Bacteriol. 1975 Apr;122(1):110–119. doi: 10.1128/jb.122.1.110-119.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J. Transport and utilization of D-methionine and other methionine sources in Escherichia coli. J Bacteriol. 1977 Jan;129(1):207–216. doi: 10.1128/jb.129.1.207-216.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J. Transport systems for L-methionine in Escherichia coli. J Bacteriol. 1974 Jan;117(1):232–241. doi: 10.1128/jb.117.1.232-241.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Watson W. J. Methionine transport in Escherichia coli: physiological and genetic evidence for two uptake systems. J Bacteriol. 1974 Aug;119(2):401–409. doi: 10.1128/jb.119.2.401-409.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Winkler H. H. Energy coupling for methionine transport in Escherichia coli. J Bacteriol. 1975 Sep;123(3):985–991. doi: 10.1128/jb.123.3.985-991.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Horikoshi K. Sodium ion-stimulated alpha-[1-14C]aminoisobutyric acid uptake in alkalophilic Bacillus species. J Bacteriol. 1977 Sep;131(3):784–788. doi: 10.1128/jb.131.3.784-788.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso S. The relationship between methionine uptake and demethiolation in a methionine-utilizing mutant of Pseudomonas fluorescens UK1. J Gen Microbiol. 1976 Aug;96(2):391–394. doi: 10.1099/00221287-95-2-391. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Yearwood-Drayton V., MacDonald R. E. Light-induced glutamate transport in Halobacterium halobium envelope vesicles. I. Kinetics of the light-dependent and the sodium-gradient-dependent uptake. Biochemistry. 1976 Apr 20;15(8):1595–1603. doi: 10.1021/bi00653a001. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Montie D. B., Montie T. C. Methionine transport in Yersinia pestis. J Bacteriol. 1975 Oct;124(1):296–306. doi: 10.1128/jb.124.1.296-306.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntsälä P., Laakso S., Nurmikko V. Observations on methionine transport in Pseudomonas fluorescens UK1. J Gen Microbiol. 1974 Sep;84(1):19–27. doi: 10.1099/00221287-84-1-19. [DOI] [PubMed] [Google Scholar]
- Niiya S., Moriyama Y., Futai M., Tsuchiya T. Cation coupling to melibiose transport in Salmonella typhimurium. J Bacteriol. 1980 Oct;144(1):192–199. doi: 10.1128/jb.144.1.192-199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg G. D., Furlong C. E. Resolution of the multiplicity of the glutamate and aspartate transport systems of Escherichia coli. J Biol Chem. 1977 Dec 25;252(24):9055–9064. [PubMed] [Google Scholar]
- Shiio I., Miyajima R., Kashima N. Na+-dependent transport of threonine in Brevibacterium flavum. J Biochem. 1973 Jun;73(6):1185–1193. doi: 10.1093/oxfordjournals.jbchem.a130190. [DOI] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Functions of Na+ and K+ in the active transport of -aminoisobutyric acid in a marine pseudomonad. J Biol Chem. 1971 Jun 25;246(12):4066–4074. [PubMed] [Google Scholar]
- Tsuchiya T., Hasan S. M., Raven J. Glutamate transport driven by an electrochemical gradient of sodium ions in Escherichia coli. J Bacteriol. 1977 Sep;131(3):848–853. doi: 10.1128/jb.131.3.848-853.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


