Abstract
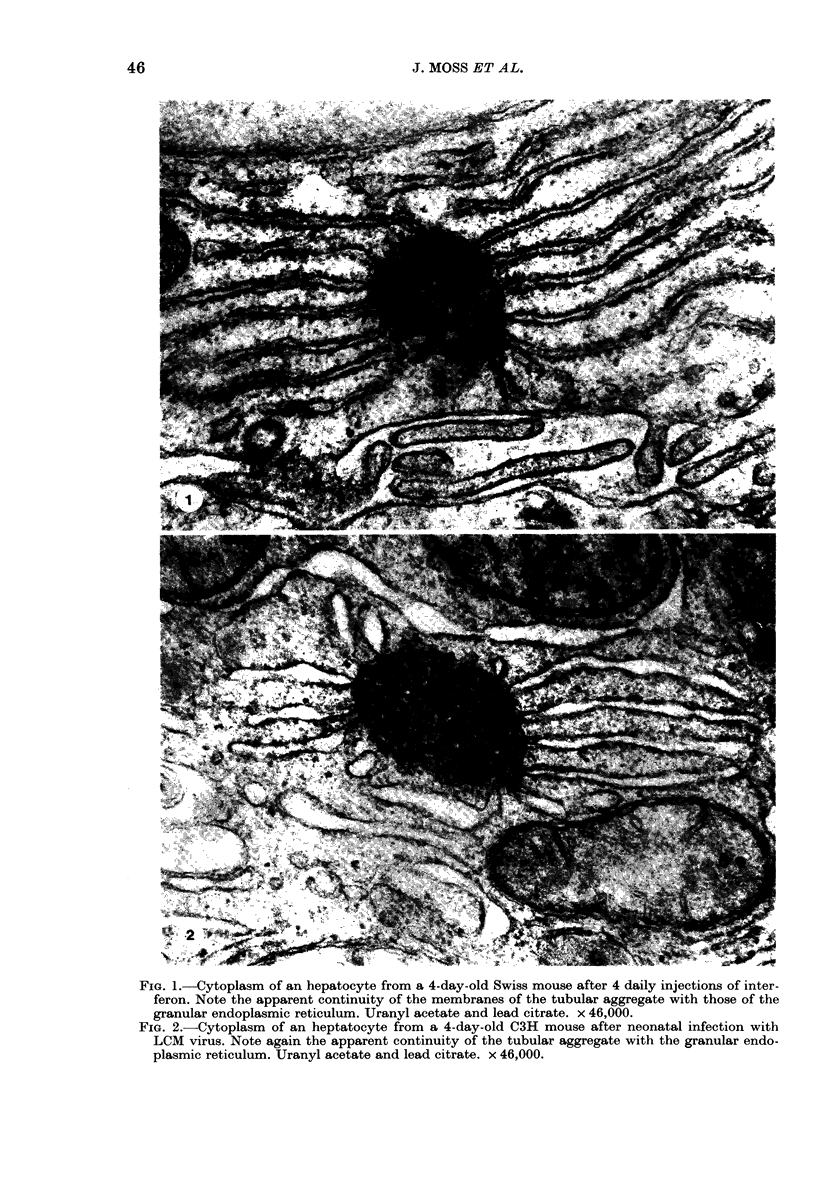
An ultrastructural examination of livers from newborn mice, injected with potent partially purified or highly purified mouse interferon or with lymphocytic choriomeningitis (LCM) virus, has revealed the presence of tubular aggregates associated with the granular endoplasmic reticulum in the cytoplasm of hepatocytes after either treatment. Thus the lesion was observed in A2G and Swiss mice after interferon injections. It was also seen in C3H mice after LCM infection, the liver being examined at a time when the interferonaemia in the injected mice was known to be at its peak. The aggregate resembles the tubular systems associated with the endoplasmic reticulum described in various tissues in both human and animal diseases. These observations raise the possibility that in some of the cases previously described the lesion has been interferon induced.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M. High-affinity binding of 125I-labelled mouse interferon to a specific cell surface receptor. Nature. 1980 Apr 3;284(5755):459–461. doi: 10.1038/284459a0. [DOI] [PubMed] [Google Scholar]
- Chandra S. Undulating tubules associated with endoplasmic reticulum in pathologic tissues. Lab Invest. 1968 Apr;18(4):422–428. [PubMed] [Google Scholar]
- DAVID-FERREIRA J. F., MANAKER R. A. AN ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF A MOUSE HEPATITIS VIRUS IN TISSUE CULTURE CELLS. J Cell Biol. 1965 Jan;24:57–78. doi: 10.1083/jcb.24.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maeyer-Guignard J., Tovey M. G., Gresser I., De Maeyer E. Purification of mouse interferon by sequential affinity chromatography on poly(U)--and antibody--agarose columns. Nature. 1978 Feb 16;271(5646):622–625. doi: 10.1038/271622a0. [DOI] [PubMed] [Google Scholar]
- Gresser I., Aguet M., Morel-Maroger L., Woodrow D., Puvion-Dutilleul F., Guillon J. C., Maury C. Electrophoretically pure mouse interferon inhibits growth, induces liver and kidney lesions, and kills suckling mice. Am J Pathol. 1981 Mar;102(3):396–402. [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Fontaine-Brouty-Boyé D., Bourali C., Thomas M. T. A comparison of the efficacy of endogenous, exogenous, and combined endogenous-exogenous interferon in the treatment of mice infected with encephalomyocarditis virus. Proc Soc Exp Biol Med. 1969 Jan;130(1):236–242. doi: 10.3181/00379727-130-33529. [DOI] [PubMed] [Google Scholar]
- Gresser I., Maury C., Tovey M., Morel-Maroger L., Pontillon F. Progressive glomerulonephritis in mice treated with interferon preparations at birth. Nature. 1976 Sep 30;263(5576):420–422. doi: 10.1038/263420a0. [DOI] [PubMed] [Google Scholar]
- Gresser I. On the varied biologic effects of interferon. Cell Immunol. 1977 Dec;34(2):406–415. doi: 10.1016/0008-8749(77)90262-3. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Maury C., Chouroulinkov I. Lethality of interferon preparations for newborn mice. Nature. 1975 Nov 6;258(5530):76–78. doi: 10.1038/258076a0. [DOI] [PubMed] [Google Scholar]
- Gresser J., Morel-Maroger L., Verroust P., Rivière Y., Guillon J. C. Anti-interferon globulin inhibits the development of glomerulonephritis in mice infected at birth with lymphocytic choriomeningitis virus. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3413–3416. doi: 10.1073/pnas.75.7.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györkey F., Min K. W., Sincovics J. G., Györkey P. Systemic lupus erythematosus and myxovirus. N Engl J Med. 1969 Feb 6;280(6):333–333. doi: 10.1056/nejm196902062800620. [DOI] [PubMed] [Google Scholar]
- Hooks J. J., Moutsopoulos H. M., Geis S. A., Stahl N. I., Decker J. L., Notkins A. L. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979 Jul 5;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rivière Y., Gresser I., Guillon J. C., Tovey M. G. Inhibition by anti-interferon serum of lymphocytic choriomeningitis virus disease in suckling mice. Proc Natl Acad Sci U S A. 1977 May;74(5):2135–2139. doi: 10.1073/pnas.74.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner F. The structural basis of altered hepatic function in viral hepatitis. Am J Med. 1970 Nov;49:658–668. doi: 10.1016/s0002-9343(70)80132-2. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. K., Feinstone S. M., Purcell R. H., Alter H. J., London W. T. Non-A, non-B hepatitis: ultrastructural evidence for two agents in experimentally infected chimpanzees. Science. 1979 Jul 13;205(4402):197–200. doi: 10.1126/science.451589. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Begon-Lours J., Gresser I. A method for the large scale production of potent interferon preparations. Proc Soc Exp Biol Med. 1974 Jul;146(3):809–815. doi: 10.3181/00379727-146-38196. [DOI] [PubMed] [Google Scholar]
- Tsiquaye K. N., Bird R. G., Tovey G., Wyke R. J., Williams R., Zuckerman A. J. Further evidence of cellular changes associated with non-A, non-B hepatitis. J Med Virol. 1980;5(1):63–71. doi: 10.1002/jmv.1890050108. [DOI] [PubMed] [Google Scholar]
- Uzman B. G., Saito H., Kasac M. Tubular arrays in the endoplasmic reticulum in human tumor cells. Lab Invest. 1971 Jun;24(6):492–498. [PubMed] [Google Scholar]




