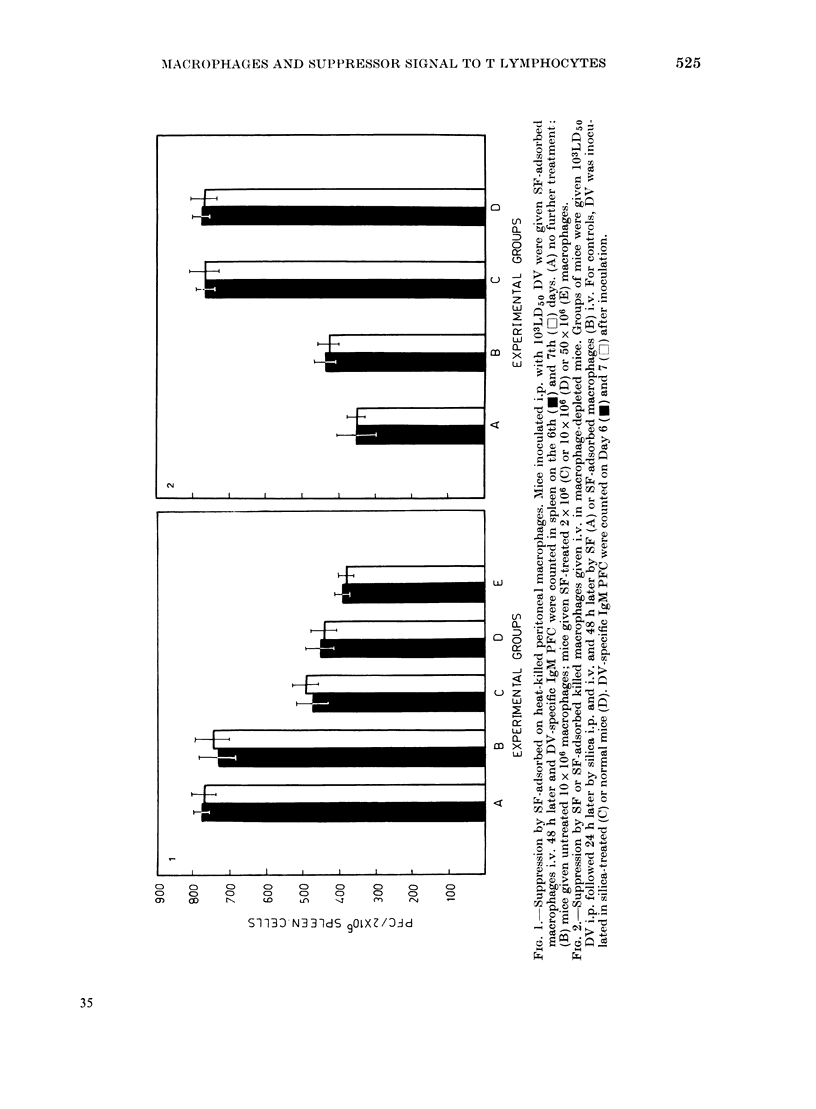
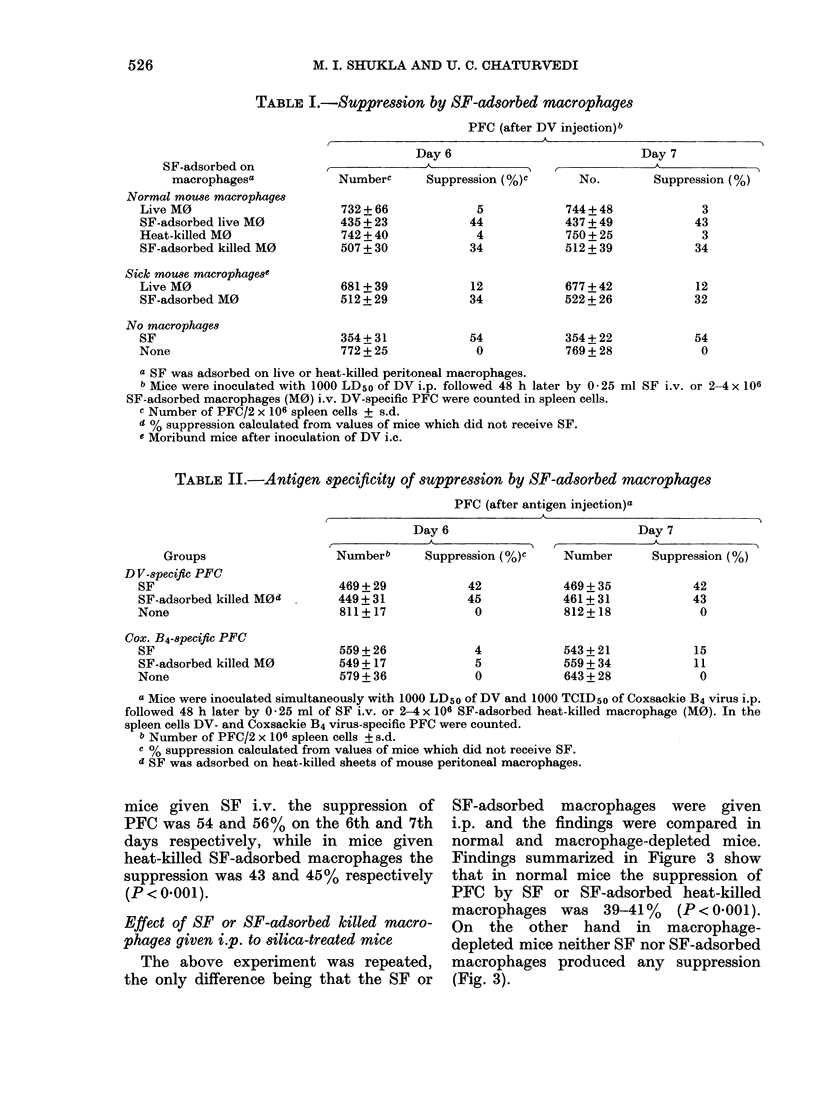
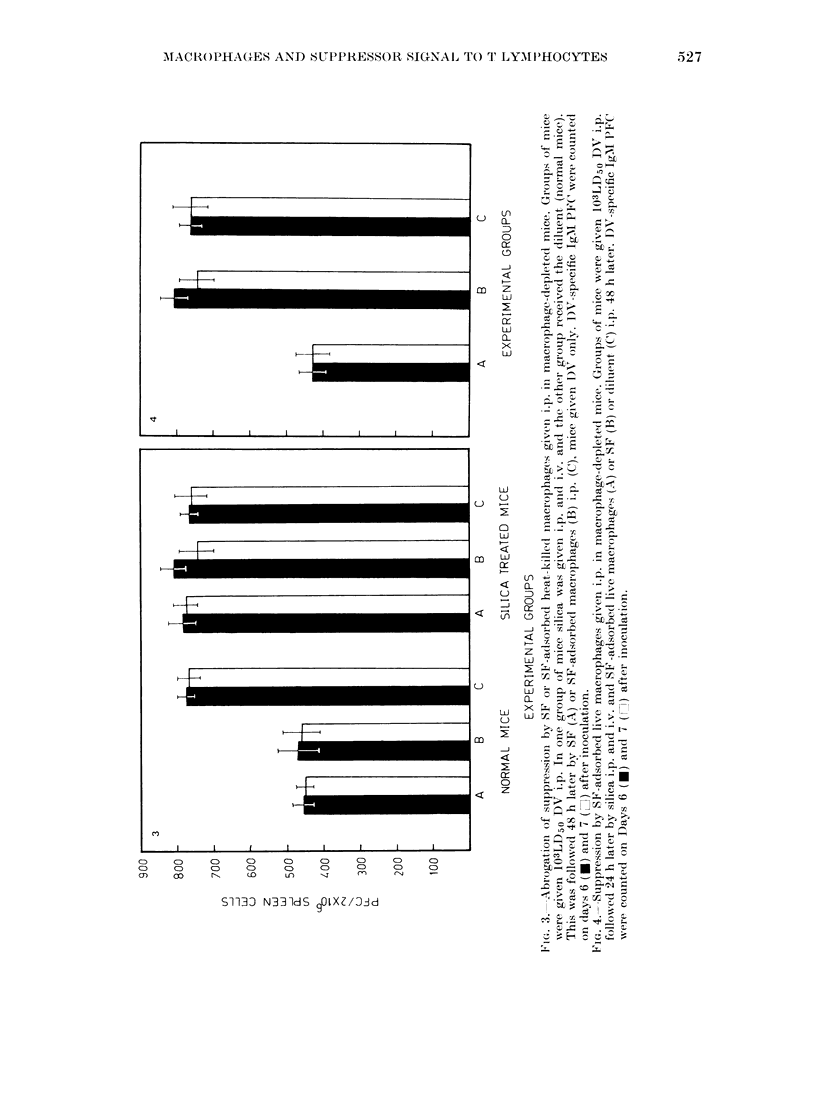
Abstract
Dengue type 2 virus (DV)-induced suppressor T cells (Ts1) produce a soluble suppressor factor (SF) which stimulates a subpopulation of T lymphocytes (Ts2) to produce prostaglandin which suppresses DV-specific IgM antibody plaque forming cells (PFC) in vivo and in vitro. The present study was undertaken to investigate the intermediary role of macrophages in transmission of signal from Ts1 to Ts2. The SF is adsorbed on live or heat-killed mouse peritoneal macrophages and transmits DV-specific PFC in spleen after i.p. or i.v. inoculation in DV-primed mice. The suppression is antigen-specific. SF or SF-adsorbed heat-killed macrophages failed to transmit suppression in silica-treated macrophage-depleted mice when injected i.p., while SF-adsorbed live macrophages could transmit suppression. In macrophage-depleted mice suppression could be transmitted by SF-adsorbed heat-killed macrophages on i.v. inoculation. It was therefore concluded that live macrophage-killed cells are also essential for transmission of suppressor signal by SF from Ts1 to Ts2 in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal D. K., Tandon P., Chaturvedi U. C., Kumar A. Biochemical study of certain enzymes and metabolites of the carbohydrate metabolism in the skeletal muscle of the dengue virus-infected mice. J Gen Virol. 1978 Aug;40(2):399–408. doi: 10.1099/0022-1317-40-2-399. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Bhargava A., Mathur A. Production of cytotoxic factor in the spleen of dengue virus-infected mice. Immunology. 1980 Aug;40(4):665–671. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Dalakoti H., Mathur A. Characterization of the cytotoxic factor produced in the spleen of dengue virus-infected mice. Immunology. 1980 Oct;41(2):387–392. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Gulati L., Mathur A. Inhibition of E-rosette formation and phagocytosis by human blood leucocytes after treatment with the dengue virus-induced cytotoxic factor. Immunology. 1982 Apr;45(4):679–685. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Mathur A., Tandon P., Natu S. M., Rajvanshi S., Tandon H. O. Variable effect on peripheral blood leucocytes during JE virus infection of man. Clin Exp Immunol. 1979 Dec;38(3):492–498. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Mathur K. R., Gulati I., Mathur A. Target lymphoid cells for the cytotoxic factor produced in the spleen of dengue virus-infected mice. Immunol Lett. 1981 Apr;3(1):13–16. doi: 10.1016/0165-2478(81)90088-2. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Shukla M. I., Mathur A. Thymus-dependent lymphocytes of dengue virus-infected mice spleens mediate suppression through prostaglandin. Immunology. 1981 Jan;42(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Shukla M. I., Mathur K. R., Mathur A. Dengue virus-induced cytotoxic factor suppresses immune response of mice to sheep erythrocytes. Immunology. 1981 Jun;43(2):311–316. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Shukla M. I. [Characterization of the suppressor factor produced in the spleen of dengue virus-infected mice]. Ann Immunol (Paris) 1981 May-Jun;132C(3):245–255. doi: 10.1016/0769-2625(81)90075-1. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon H. O., Mathur A. Control of in vitro and in vivo spread of coxsackievirus B4 infection by sensitized spleen cells and antibody. J Infect Dis. 1978 Aug;138(2):181–190. doi: 10.1093/infdis/138.2.181. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon P., Mathur A. Effect of immunosuppression on dengue virus infection in mice. J Gen Virol. 1977 Sep;36(3):449–458. doi: 10.1099/0022-1317-36-3-449. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon P., Mathur A., Kumar A. Host defence mechanisms against dengue virus infection of mice. J Gen Virol. 1978 May;39(2):293–302. doi: 10.1099/0022-1317-39-2-293. [DOI] [PubMed] [Google Scholar]
- Gulati L., Chaturvedi U. C., Mathur A. Depressed macrophage functions in dengue virus-infected mice: role of the cytotoxic factor. Br J Exp Pathol. 1982 Apr;63(2):194–202. [PMC free article] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke E. J., Halstead S. B., Allison A. C., Platts-Mills T. A. Specific lethality of silica for human peripheral blood mononuclear phagocytes, in vitro. J Immunol Methods. 1978;19(2-3):137–151. doi: 10.1016/0022-1759(78)90174-6. [DOI] [PubMed] [Google Scholar]
- Shukla M. I., Chaturvedi U. C. Cycloheximide & mitomycin C inhibited production of dengue virus-induced suppressor factor. Indian J Exp Biol. 1981 Sep;19(9):826–828. [PubMed] [Google Scholar]
- Shukla M. I., Chaturvedi U. C. Dengue virus-induced suppressor factor stimulates production of prostaglandin to mediate suppression. J Gen Virol. 1981 Oct;56(Pt 2):241–249. doi: 10.1099/0022-1317-56-2-241. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon P., Chaturvedi U. C., Mathur A. Dengue virus-induced thymus-derived suppressor cells in the spleen of mice. Immunology. 1979 Dec;38(4):653–658. [PMC free article] [PubMed] [Google Scholar]
- Tandon P., Chaturvedi U. C., Mathur A. Differential depletion of T lymphocytes in the spleen of dengue virus-infected mice. Immunology. 1979 May;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]


