Abstract
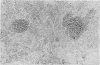
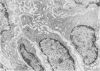
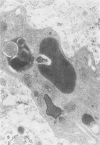
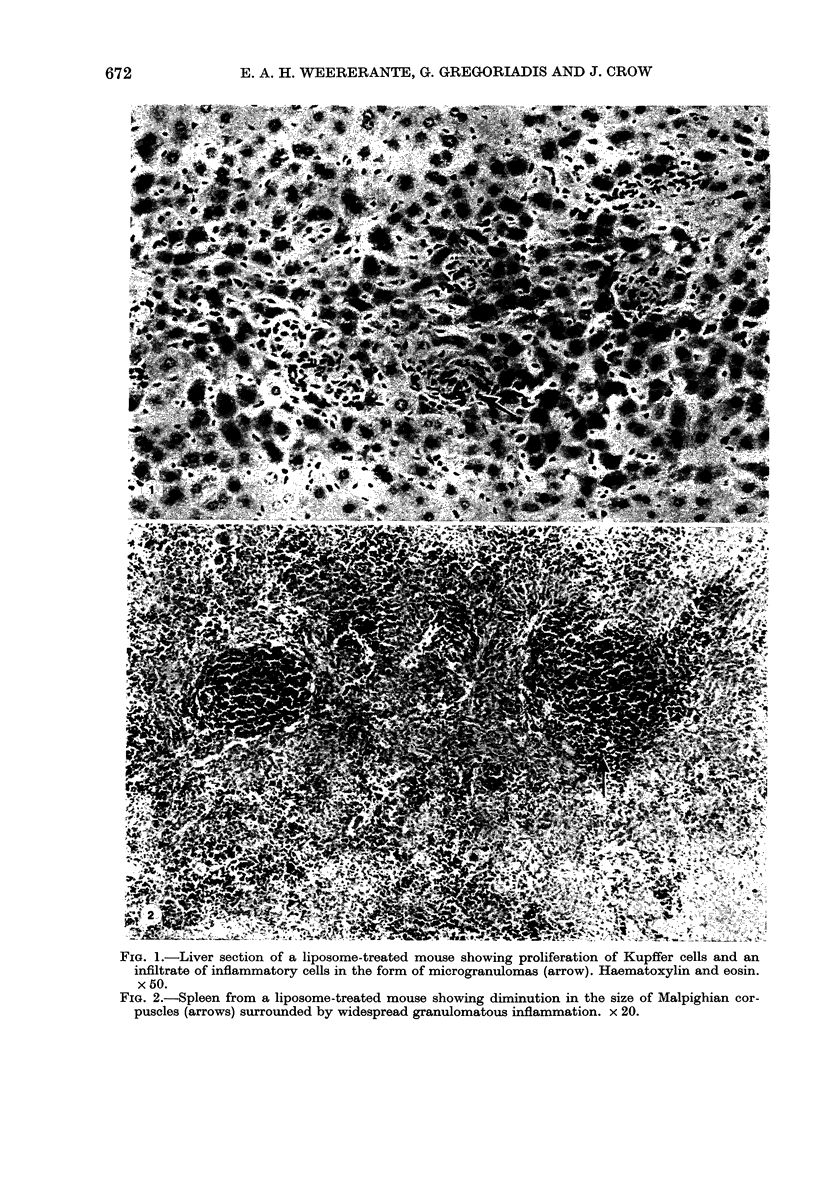
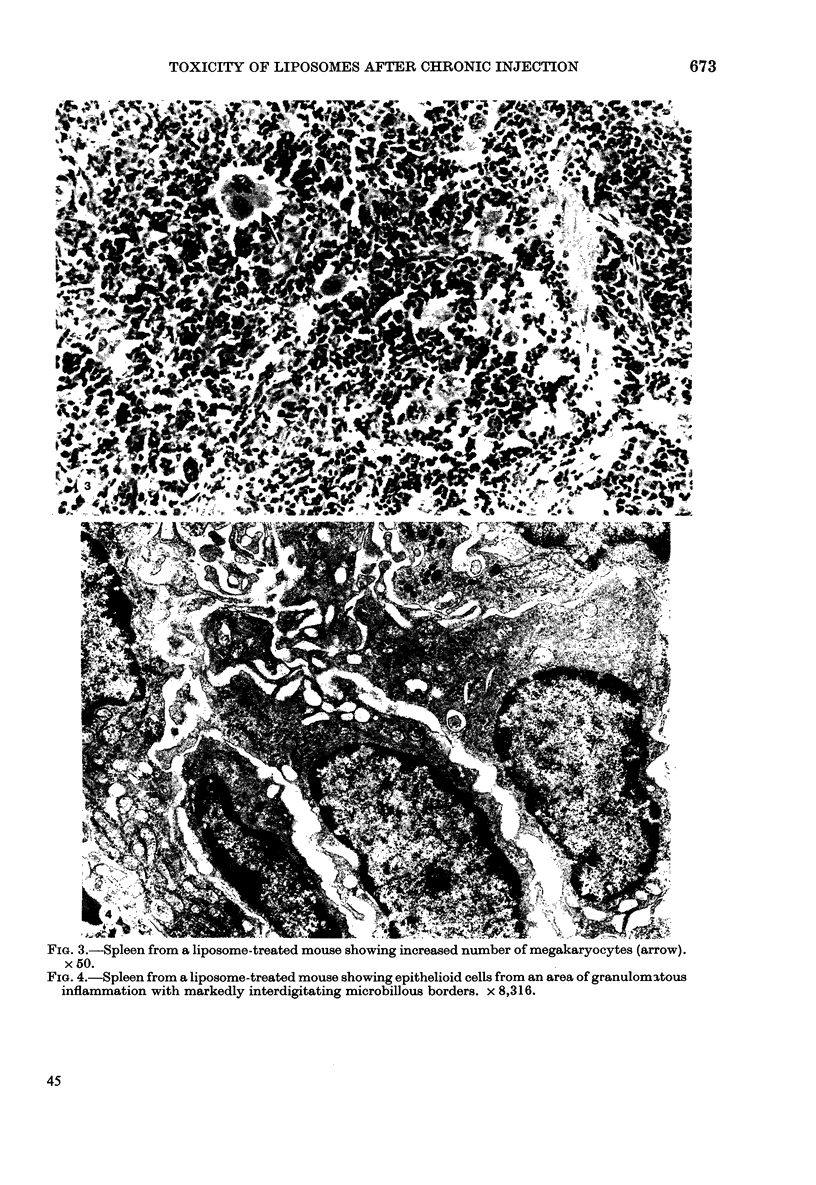
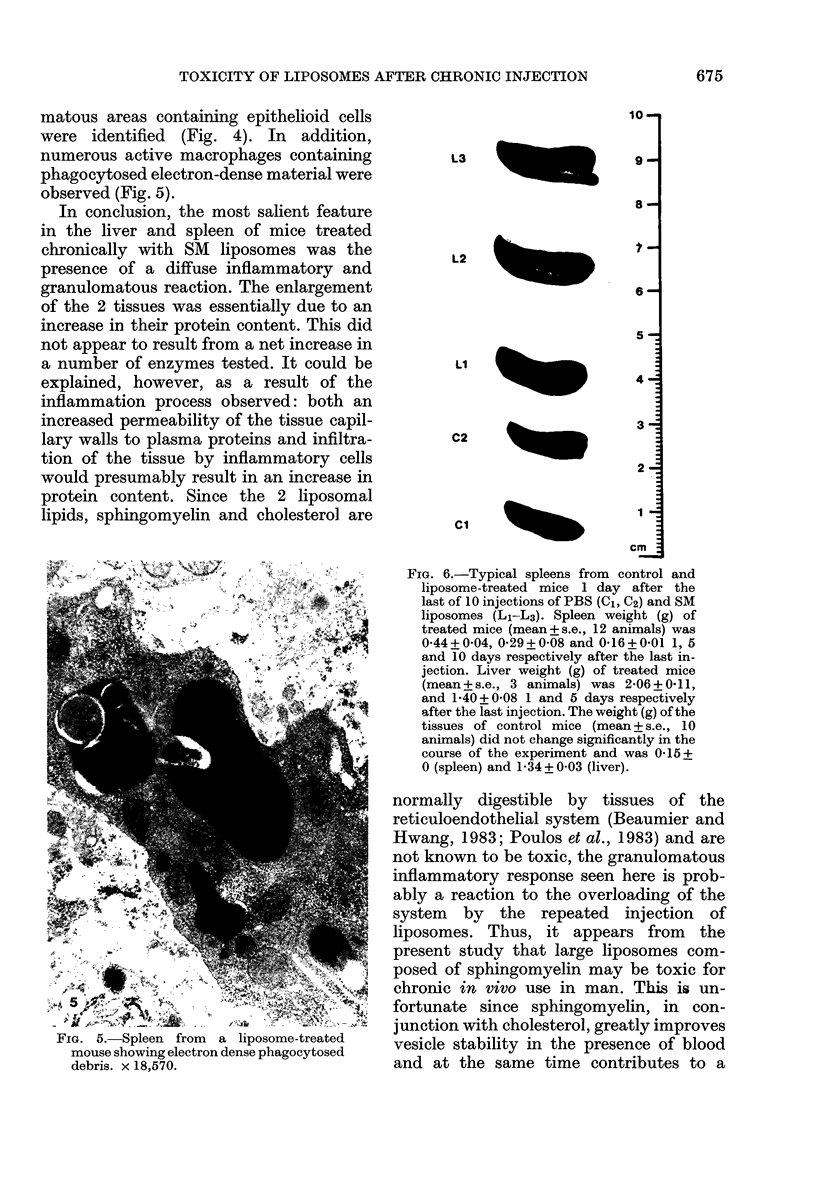
Chronic treatment (10 i.p. injections over 20 days) of Balb/c mice with SM liposomes led to 50 and 300% enlargement of the liver and spleen respectively. No such effect was observed after similar treatment with PC liposomes. Biochemical analysis of the enlarged tissues showed no significant changes in the concentrations of glycolipid, phospholipid and certain hydrolytic enzymes. However, the increase in tissue size was paralleled by an increase in protein content. Light and electron microscopy studies of the enlarged tissues revealed an increase in the number of Kupffer cells and collections of inflammatory cells in the liver and widespread granulomatous inflammation in the spleen. We conclude that SM liposomes, although probably toxic for use as a drug carrier, may serve as a model agent in the study of tissue granulomatous inflammation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaumier P. L., Hwang K. J. Effects of liposome size on the degradation of bovine brain sphingomyelin/cholesterol liposomes in the mouse liver. Biochim Biophys Acta. 1983 May 26;731(1):23–30. doi: 10.1016/0005-2736(83)90393-0. [DOI] [PubMed] [Google Scholar]
- Braidman I. P., Gregoriadis G. Rapid partial purification of placental glucocerebroside beta-glucosidase and its entrapment in liposomes. Biochem J. 1977 May 15;164(2):439–445. doi: 10.1042/bj1640439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gregoriadis G. Targeting of drugs: implications in medicine. Lancet. 1981 Aug 1;2(8240):241–246. doi: 10.1016/s0140-6736(81)90486-4. [DOI] [PubMed] [Google Scholar]
- Kampine J. P., Kanfer J. N., Gal A. E., Bradley R. M., Brady R. O. Response of sphingolipid hydrolases in spleen and liver to increased erythrocytorhexis. Biochim Biophys Acta. 1967 Feb 14;137(1):135–139. doi: 10.1016/0005-2760(67)90016-1. [DOI] [PubMed] [Google Scholar]
- Kirby C., Clarke J., Gregoriadis G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem J. 1980 Feb 15;186(2):591–598. doi: 10.1042/bj1860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby C., Gregoriadis G. Plasma-induced release of solutes from small unilamellar liposomes is associated with pore formation in the bilayers. Biochem J. 1981 Oct 1;199(1):251–254. doi: 10.1042/bj1990251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Poulos A., Shankaran P., Jones C. S., Callahan J. W. Enzymatic hydrolysis of sphingomyelin liposomes by normal tissues and tissues from patients with Niemann-Pick disease. Biochim Biophys Acta. 1983 May 16;751(3):428–431. doi: 10.1016/0005-2760(83)90302-8. [DOI] [PubMed] [Google Scholar]
- Purkiss P., Hughes R. C., Watts R. W. Screening for urinary oligosaccharides: a quantitative procedure. Clin Chem. 1978 Apr;24(4):669–674. [PubMed] [Google Scholar]
- Senior J., Gregoriadis G. Is half-life of circulating liposomes determined by changes in their permeability? FEBS Lett. 1982 Aug 16;145(1):109–114. doi: 10.1016/0014-5793(82)81216-7. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D. E., Sweeley C. C. Quantitative determination of the neutral glycosyl ceramides in human blood. J Lipid Res. 1967 Nov;8(6):621–630. [PubMed] [Google Scholar]