Abstract
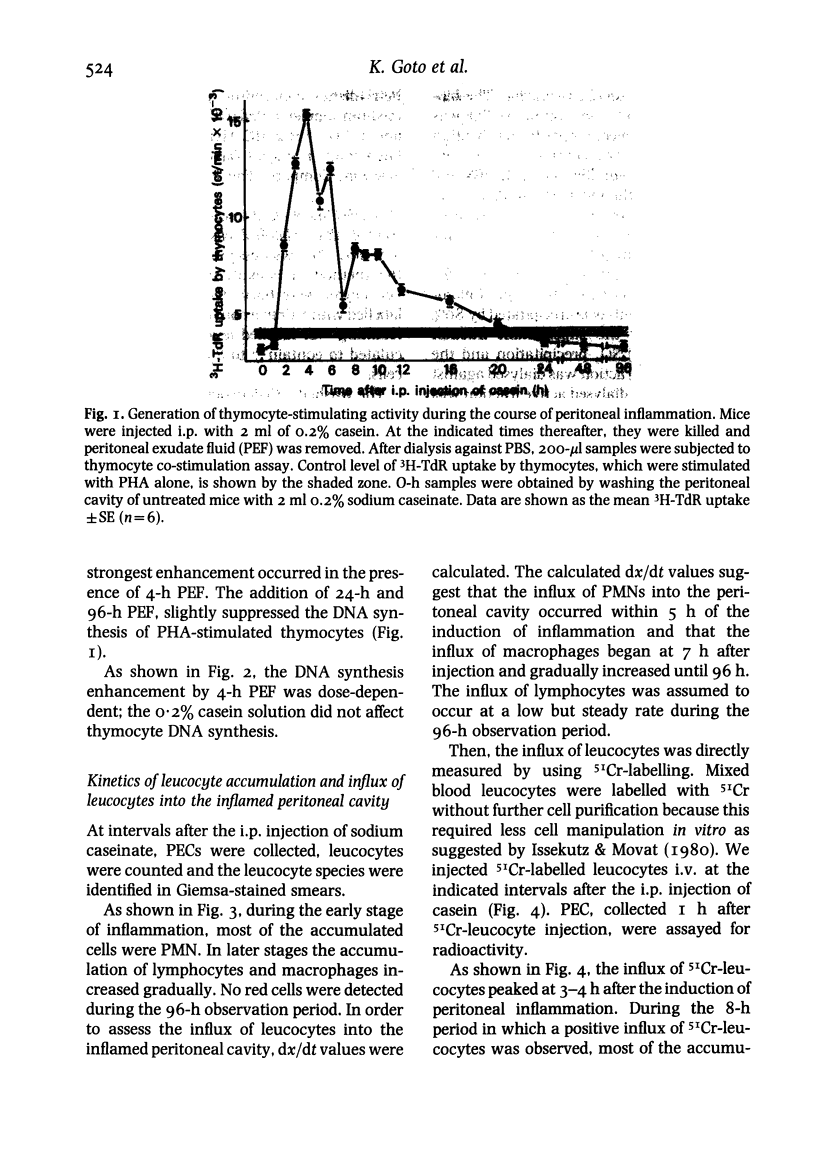
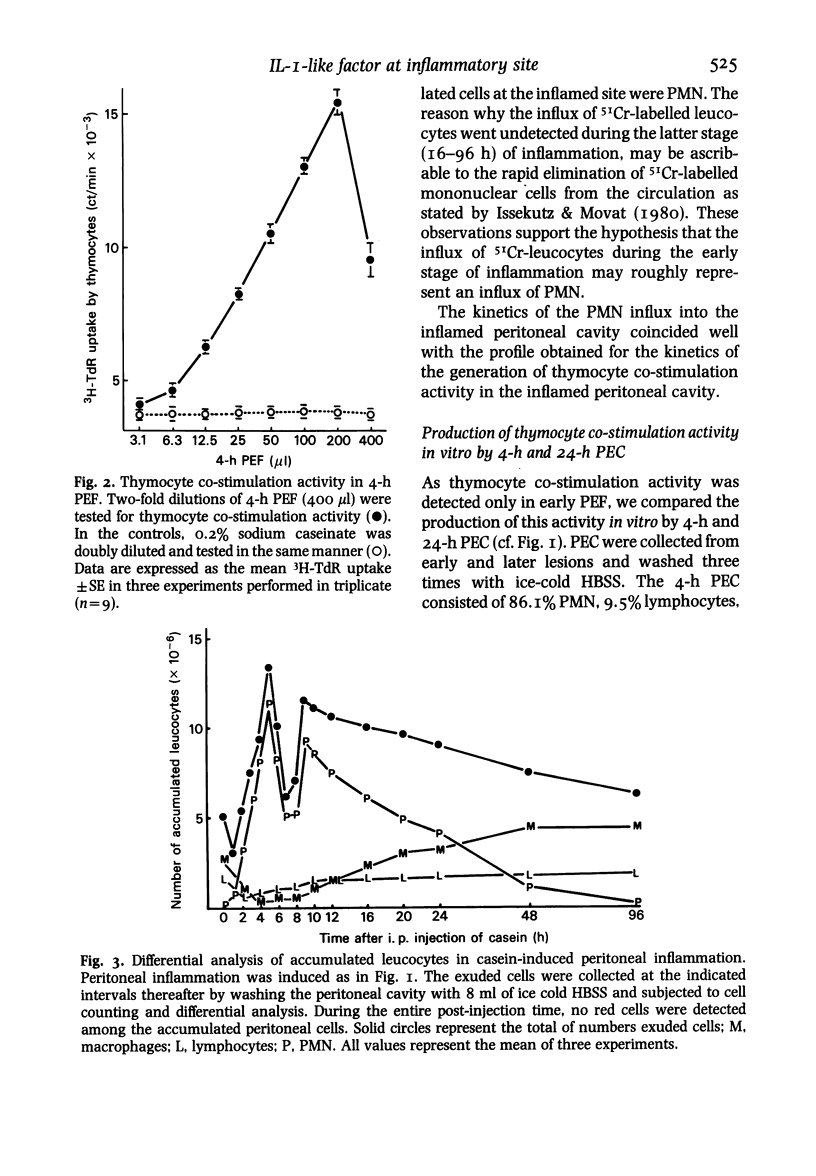
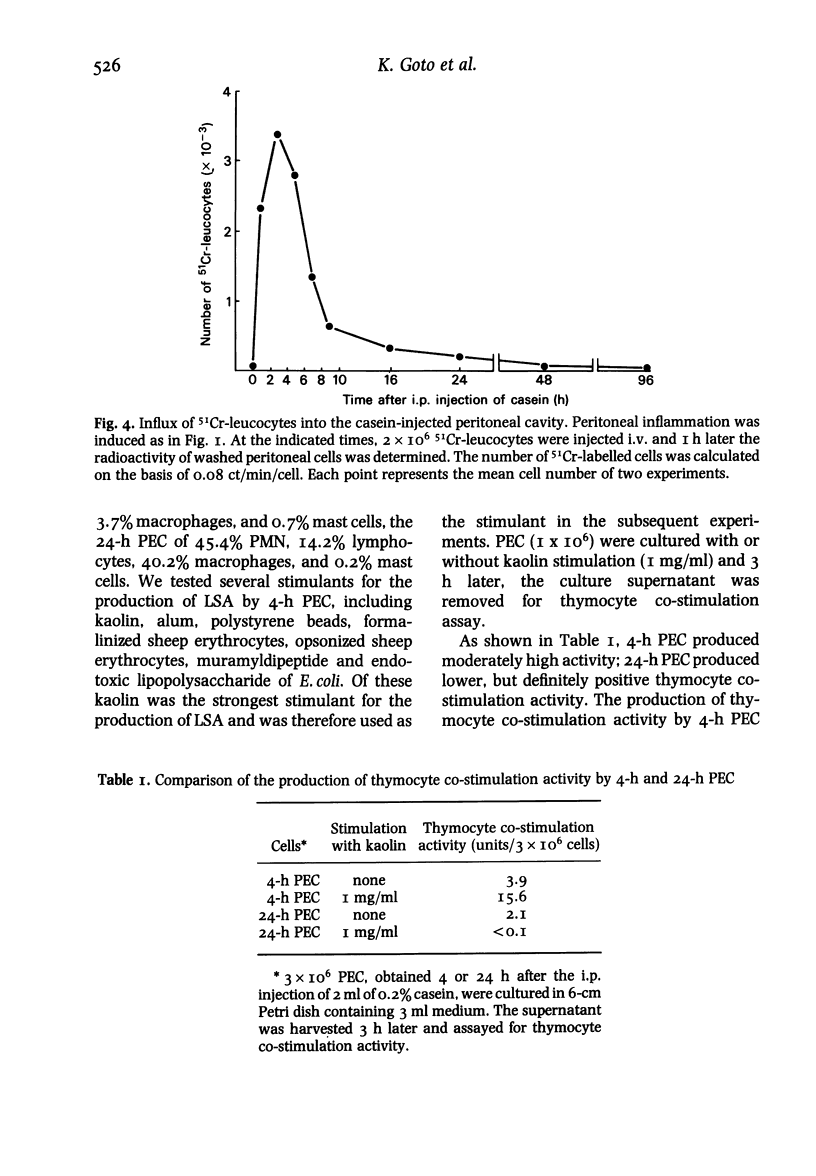
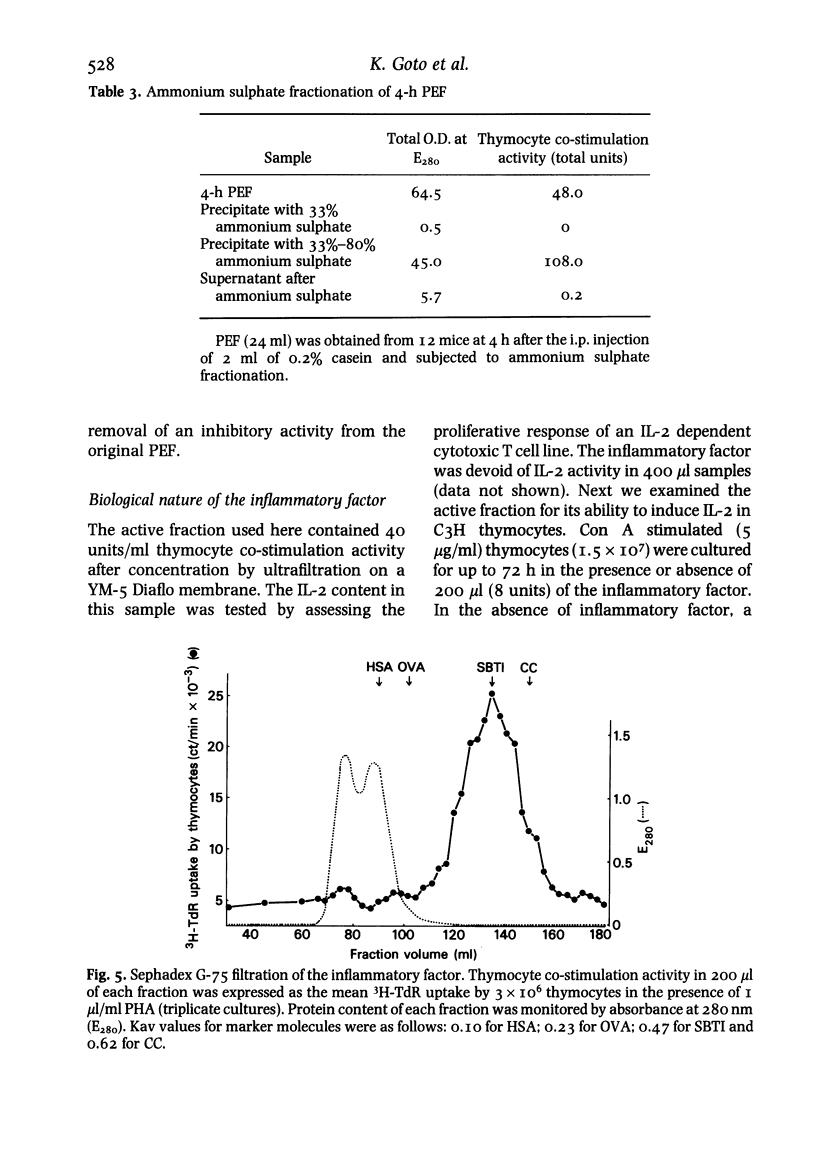
Using a mouse thymocyte co-stimulation assay, we demonstrated thymocyte-stimulating activity in murine peritoneal fluid obtained from the early stage (3 to 9 h) of casein-induced inflammation. This early inflammatory stage coincided with the time at which an influx of polymorphonuclear leucocytes (PMN) into the inflamed site was observed. Similar thymocyte-stimulating activity was produced in vitro by PMN purified from 4-h peritoneal exudate but not by purified PMN obtained at a later stage (24 h) of the inflammation. The inflammatory factor was interleukin (IL)-I-like; it was devoid of IL-2 activity when tested with IL-2-dependent cells. It could stimulate murine thymocytes to produce IL-2. On a Sephadex G-75 column, the factor was eluted between the molecular sizes of 10 000 and 30 000; its peak activity was at 21 000. The factor mainly consisted of two (pI 6.5 and pI 5.0) iso-electrophoretically different factors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Economou J. S., Shin H. S. Lymphocyte-activating factor. I. Generation and physicochemical characterization. J Immunol. 1978 Oct;121(4):1446–1452. [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D. F., Murphy P. A., Windle B. E. Failure of rabbit neutrophils to secrete endogenous pyrogen when stimulated with staphylococci. J Exp Med. 1980 Jun 1;151(6):1360–1371. doi: 10.1084/jem.151.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ise K., Nakamura S., Ohkawara S., Yoshinaga M. DNA synthesis-potentiating activity on mouse thymocytes of synovial fluid of rheumatoid arthritis patients. Acta Pathol Jpn. 1982 May;32(3):491–503. doi: 10.1111/j.1440-1827.1982.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The in vivo quantitation and kinetics of rabbit neutrophil leukocyte accumulation in the skin in response to chemotactic agents and Escherichia coli. Lab Invest. 1980 Mar;42(3):310–317. [PubMed] [Google Scholar]
- Mizel S. B. Studies on the purification and structure-functional relationships of murine lymphocyte activating factor (Interleukin 1). Mol Immunol. 1980 May;17(5):571–577. doi: 10.1016/0161-5890(80)90155-8. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Nakamura S., Goto F., Goto K., Yoshinaga M. Physicochemical characterization of a PMN-derived soluble fraction that enhances lymphocyte DNA synthesis. J Immunol. 1982 Jun;128(6):2614–2621. [PubMed] [Google Scholar]
- Nakayama S., Rodriguez-Pinzon J., Nakamura S., Yoshinaga M. A possible role of PMN in a casein-induced enhancement of PFC response to sheep erythrocytes in mice. Immunology. 1982 Apr;45(4):669–677. [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A., Rosenthal A. S. Adherent cell function in murine T-lymphocyte antigen recognition. IV. Enhancement of murine T-cell antigen recognition by human leukocytic pyrogen. J Exp Med. 1979 Sep 19;150(3):709–714. doi: 10.1084/jem.150.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer T. L., Bretz U., Baggiolini M. In vitro stimulation of lymphocytes by neutral proteinases from human polymorphonuclear leukocyte granules. J Exp Med. 1976 Oct 1;144(4):863–872. doi: 10.1084/jem.144.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]


