It is a curious and intriguing situation that mammalian sperm are introduced into the female reproductive tract in an infertile state and must be educated there before they are able to fertilize oocytes. The recognition that mammalian sperm must first be switched into a competent state, or capacitated, was exploited in the development of in vitro fertilization methods and has proved essential for the dependent technologies of clinical assisted reproduction and infertility treatments. Yet many aspects of the mechanisms of capacitation remain unclear (for recent reviews, see refs. 1 and 2). In a recent issue of PNAS, Xu et al. (3) advanced our understanding of capacitation by showing that CFTR, the cystic fibrosis transmembrane regulator, plays an essential role in some aspects of this process.
During capacitation there is a complex alteration of the biochemical, biophysical, and cell biological properties of sperm. These include (but are not restricted to) alterations in membrane potential and membrane sterol content, a rise in pHi and changes in other intracellular ion activities, and the enhanced tyrosine phosphorylation of an array of sperm proteins. As a result of this reprogramming, sperm become competent to fertilize (1, 2).
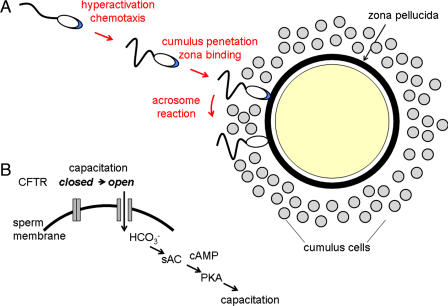
Capacitation is now understood as a physiological transformation that renders sperm better able to reach the oocyte surface. In this regard, the task confronting the mammalian sperm may be summarized as follows (Fig. 1A). Fertilization typically occurs in the ampulla of the oviduct. Sperm must reach the vicinity of the oocyte; penetrate between the several thousand cumulus oophorus cells; contact and penetrate the oocyte extracellular matrix, or zona pellucida; and finally adhere to and fuse with the oocyte plasma membrane (2). Uncapacitated sperm carry out these tasks inefficiently and fail at various steps in the process.
Fig. 1.
Capacitation and fertilization. (A) As a result of capacitation, sperm develop hyperactivated motility, the ability to respond to chemotactic signals and acrosome reaction-inducing signals. Capacitated sperm penetrate the cumulus and reach the zona pellucida. Contact with the zona pellucida triggers acrosome reactions, permitting sperm to penetrate to the oocyte surface and fuse with the oocyte. Multiple steps in this process are controlled by capacitation. (B) Simple model of CFTR function during capacitation, in which HCO3− entry through CFTR stimulates an sAC/PKA cascade leading to the activation of downstream effectors and to capacitation. It is unresolved whether this pathway can fully account for the modulation of sperm function during capacitation and of how CFTR conductance is controlled to drive this cascade.
Three modifications in the functional properties of sperm occur during capacitation that may contribute to efficient fertilization.
Sperm develop the ability to respond to chemotactic signals. Chemotactic activities are associated with oocytes, cumulus cells, and possibly other components of the female reproductive tract (4), and functional chemotactic signaling pathways are present in sperm (5). The molecular nature of the active factor(s) has not yet been determined. This pathway may assist sperm guidance to the site of fertilization (4).
Flagellar motility switches from an activated mode, characterized by symmetric beating with shallow bends, to a hyperactivated mode in which the beat is asymmetric and exhibits deep bends. Hyperactivated motility may be required for sperm ascent of the oviduct and penetration through the zona pellucida (6, 7).
Sperm acquire the ability to interact with oocytes. To penetrate the zona pellucida, sperm must first complete an exocytotic event, the acrosome reaction. This is triggered by one of the zona pellucida glycoproteins, ZP3, following sperm contact. The efficiency of the signal transducing pathways in sperm that are activated by ZP3 and drive exocytosis is enhanced as a result of capacitation, resulting in a robust secretory response when capacitated sperm contact either the zona pellucida or purified ZP3. Furthermore, the acrosome reaction is a prerequisite for penetration of the zona pellucida and also for fusion with oocytes, and so these aspects of gamete interaction are indirectly regulated by capacitation (2).
Unresolved is the question of how those biochemical and biophysical changes result in the maturation of sperm functional capacity. Previous in vitro studies have consistently pointed to the importance of HCO3− as a medium component that, in concert with Ca2+ and a cholesterol-binding element (usually BSA), is required for capacitation (1, 2). One model of HCO3− action has emerged from recent studies. Mammalian sperm are enriched in the atypical soluble adenylyl cyclase (sAC), which is not regulated by G proteins but rather by HCO3− (8, 9). Downstream events following sAC activation include stimulation of protein kinase A (PKA) and lead to the enhancement of the tyrosine phosphorylation status of an array of sperm proteins (10, 11). Several lines of evidence support the importance of this pathway, including removal of HCO3− from the medium, inhibition of sAC or targeted deletion of the sAC gene, and inhibition of downstream effectors (10–12). In addition, there is circumstantial evidence for a role of HCO3− in capacitation in vivo (13).
But how is this capacitation cascade initiated? Although there is agreement that HCO3− is essential, there was no consensus as to how intracellular levels of this anion were regulated. In fact, given the presence of carbonic anhydrase in sperm (14), it could plausibly be argued that the elevation of pHi that accompanies capacitation (1, 2) could generate intracellular HCO3− in situ in the absence of anion influx. This situation has been clarified by the work of Xu et al. (3) on CFTR. Although initially described as a Cl− channel, it is understood that CFTR can both directly conduct an HCO3− current and interact with other HCO3− transport pathways (15). Inhibition of CFTR in sperm results in a failure of capacitation, an apparent reduction in HCO3− influx, reduced cAMP responses, and the loss of a number of the anticipated downstream targets of HCO3−/cAMP. In addition, heterozygote Cftr+/− male mice show reduced fertility in vivo and in vitro (3). These observations indicate that CFTR may drive some early events of capacitation (Fig. 1B).
During capacitation there is a complex alteration of the biochemical, biophysical, and cell biological properties of sperm.
The mechanism of CFTR activation during capacitation has not been determined. Previous studies have established that this channel opens in response to PKA phosphorylation and that this may be regulated dynamically by protein–protein interactions (16, 17). The specific pathway that initiates CFTR opening in sperm has not been determined and might include either other HCO3− transporters (18) or basal PKA activity, but in either case the downstream activation of sAC and PKA provides an obvious amplification mechanism.
This simple model may account for certain aspects of capacitation, such as enhanced protein tyrosine phosphorylation. However, capacitation is more complex than just that. Loss of sAC activity, either through targeted gene deletion or by pharmacological inhibition, results in sperm that fail to exhibit either enhanced protein tyrosine phosphorylation or hyperactivated motility but are able to undergo a zona pellucida-evoked acrosome reaction (11, 12). Thus, HCO3− may modulate only some aspects of capacitation by acting through an sAC pathway.
An interesting but unresolved question is whether CFTR function is similarly linked to only a subset of capacitation-associated events or acts more broadly. Such broader effects, on events such as acrosome reaction responses, may indicate that CFTR action is not restricted to an sAC/PKA pathway and may point to a role of other HCO3− effectors. In addition, it should not be forgotten that the major conductance of CFTR, Cl− (16), may also play a role. In any case, the recognition that this channel may play a role in the early events of capacitation points to the elaboration and testing of new models of fertilization.
Footnotes
The authors declare no conflict of interest
See companion article on page 9816 in issue 23 of volume 104.
References
- 1.Gadella BM, Visconti PE. In: The Sperm Cell: Production, Maturation, Fertilization, Regeneration. De Jonge C, Barratt C, editors. Cambridge, UK: Cambridge Univ Press; 2006. pp. 134–169. [Google Scholar]
- 2.Florman HM, Ducibella T. In: Physiology of Reproduction. Neill JD, editor. San Diego: Elsevier; 2006. pp. 55–112. [Google Scholar]
- 3.Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, Rowlands DK, Liu GY, Zhu H, Ma ZG, Wang XF, et al. Proc Natl Acad Sci USA. 2007;104:9816–9821. doi: 10.1073/pnas.0609253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenbach M, Giojalas LC. Nat Rev Mol Cell Biol. 2006;7:276–285. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- 5.Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 6.Quill TA, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, Garbers DL. Proc Natl Acad Sci USA. 2003;100:14869–14874. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarez SS, Pacey AA. Hum Reprod Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- 8.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 10.Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Development (Cambridge, UK) 1995;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- 11.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, et al. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, van Duin M, Conti M. Dev Biol. 2006;296:353–362. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Wang XF, Zhou CX, Shi QX, Yuan YY, Yu MK, Ajonuma LC, Ho LS, Lo PS, Tsang LL, Liu Y, et al. Nat Cell Biol. 2003;5:902–906. doi: 10.1038/ncb1047. [DOI] [PubMed] [Google Scholar]
- 14.Parkkila S, Kaunisto K, Kellokumpu S, Rajaniemi H. Histochemistry. 1991;95:477–482. doi: 10.1007/BF00315743. [DOI] [PubMed] [Google Scholar]
- 15.Hug MJ, Tamada T, Bridges RJ. News Physiol Sci. 2003;18:38–42. doi: 10.1152/nips.01412.2002. [DOI] [PubMed] [Google Scholar]
- 16.Ashcroft FM. Ion Channels and Disease. San Diego: Academic; 2000. [Google Scholar]
- 17.Lee JH, Richter W, Namkung W, Kim KH, Kim E, Conti M, Lee MG. J Biol Chem. 2007;282:10414–10422. doi: 10.1074/jbc.M610857200. [DOI] [PubMed] [Google Scholar]
- 18.Demarco IA, Espinosa F, Edwards J, Sosnik J, De la Vega-Beltran JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE. J Biol Chem. 2003;278:7001–7009. doi: 10.1074/jbc.M206284200. [DOI] [PubMed] [Google Scholar]