Abstract
Diapause, the dormancy common to overwintering insects, evokes a unique pattern of gene expression. In the flesh fly, most, but not all, of the fly's heat shock proteins (Hsps) are up-regulated. The diapause up-regulated Hsps include two members of the Hsp70 family, one member of the Hsp60 family (TCP-1), at least four members of the small Hsp family, and a small Hsp pseudogene. Expression of an Hsp70 cognate, Hsc70, is uninfluenced by diapause, and Hsp90 is actually down-regulated during diapause, thus diapause differs from common stress responses that elicit synchronous up-regulation of all Hsps. Up-regulation of the Hsps begins at the onset of diapause, persists throughout the overwintering period, and ceases within hours after the fly receives the signal to reinitiate development. The up-regulation of Hsps appears to be common to diapause in species representing diverse insect orders including Diptera, Lepidoptera, Coleoptera, and Hymenoptera as well as in diapauses that occur in different developmental stages (embryo, larva, pupa, adult). Suppressing expression of Hsp23 and Hsp70 in flies by using RNAi did not alter the decision to enter diapause or the duration of diapause, but it had a profound effect on the pupa's ability to survive low temperatures. We thus propose that up-regulation of Hsps during diapause is a major factor contributing to cold-hardiness of overwintering insects.
Keywords: cold tolerance, overwintering, stress proteins, RNAi
Winter poses a major challenge for insects. To be successful in a highly seasonal, temperate zone environment, insects must restrict growth and reproduction to a few months during the summer and survive the remainder of the year without feeding while confronting the rigors of winter. The shortening of day length in late summer is widely exploited as an environmental token signaling the advent of winter. For most insects, short day length evokes a stage-specific developmental arrest, known as diapause (1–3). Diapause represents an alternative developmental pathway prompted by unique patterns of gene expression that result in the sequestration of nutrient reserves, suppression of metabolism, a halt or slowing of development, and the acquisition of increased tolerance to environmental stresses such as low temperature (4).
Although a few insect species are freeze-tolerant, the majority cannot tolerate body freezing and, instead, have evolved mechanisms to avoid freezing (5). The contributors best known to prevent freezing include polyols such as glycerol and the insect blood sugar trehalose that function colligatively to depress the body's supercooling point and noncolligatively to stabilize proteins and cellular membranes (6). A few insects also have the capacity to synthesize antifreeze proteins, first described from Antarctic fish (7), that function noncolligatively in the hemolymph to decrease the insect's supercooling point (8). Cytoskeletal modifications, including alterations that enhance elasticity of the cell membrane to promote function at low temperature, are likewise widely exploited by overwintering insects to prevent cold-induced damage (9–12).
In this study, we propose that another class of protective agents, the heat shock proteins (Hsps), also contribute significantly to the overwintering cold tolerance of insects. The Hsps are a group of remarkably well described proteins that are commonly expressed in response to environmental stress. Originally described from Drosophila as a response to high temperature, and hence their name, Hsps are up-regulated by diverse stresses, including cold shock, desiccation, anoxia, and exposure to a wide range of chemicals including heavy metals, ethanol, and other contaminants (13–15). They function as molecular chaperones during periods of stress by binding to other proteins, thereby ameliorating the detrimental effects of misfolding and then promoting the return of these proteins to their native conformations when favorable conditions again prevail. In most cases, the genes encoding these proteins are rapidly up-regulated at the onset of the stress, concurrent with the down-regulation of most other genes. When more favorable conditions return, the Hsps are again down-regulated, a feature that is essential because expression of Hsps during nonstress conditions can lead to deleterious effects, including retardation and cessation of development (16).
For several years, we have known that Hsp23, a small Hsp (17), and Hsp70 (18) are developmentally up-regulated during the overwintering pupal diapause of the flesh fly, Sarcophaga crassipalpis, but several questions remain unanswered. Are additional Hsps involved in the diapause response? Does this link of Hsp expression with diapause occur in other species? What function do these Hsps serve during diapause? In this report, we review the linkage between diapause and Hsp expression in the flesh fly, present evidence that quite a few additional Hsps are diapause up-regulated, show that many additional insect species also up-regulate Hsps during diapause, and use RNAi to demonstrate that suppression of the Hsps results in a loss of cold tolerance during winter diapause. We thus argue that Hsp expression is a vital component of the overwintering defense strategy of many temperate zone insects.
Results and Discussion
Up-Regulation of Hsps During Fly Diapause.
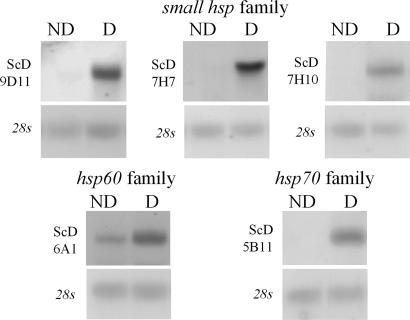
In earlier studies, we reported that both a small Hsp (Hsp23) and Hsp70 (now designated as Hsp70A) are highly up-regulated during pupal diapause in S. crassipalpis (17–19). This developmental up-regulation of Hsps occurs simply because the pupa enters diapause and is not a function of temperature stress. Unlike the stress response where there is a nearly global shut down in the expression of other genes when the Hsps are turned on, the Hsps are expressed concurrently with other genes during diapause. Attempts to further boost expression of the Hsps with temperature shocks or other stresses have not been successful; thus, these two Hsps appear to be maximally expressed during diapause. During diapause, a cognate of Hsp70, Hsc70, remains unchanged in expression (18), and Hsp90 is down-regulated (20). Thus, Hsp expression during diapause provides an interesting contrast to the typical stress response in which all Hsps are up-regulated. In addition to Hsp23 and Hsp70A, our current suppressive subtractive hybridization (SSH) analysis revealed several previously unknown Hsps that are diapause up-regulated (Table 1): three new members of the small Hsp family, a member of the Hsp60 family, a new member of the Hsp70 family, and a putative pseudogene belonging to the small Hsp family.
Table 1.
Hsps identified in the flesh fly, S crassipalpis, and their expression in relation to the fly's overwintering pupal diapause
| Hsp | GenBank accession no. | Diapause expression | Ref. |
|---|---|---|---|
| Hsp90 | AF261773 | Down | 20 |
| Hsp70A | AF107338 | Up | 18 |
| Hsp70B | EF103580 | Up | This study |
| Hsc70 | AF107339 | Unchanged | 18 |
| Hsp60 | EF103581 | Up | This study |
| Hsp25 | EF103577 | Up | This study |
| Hsp23 | U96099 | Up | 17 |
| Hsp23 pseudogene | AF156162 | Up | This study |
| Hsp18 | EF103578 | Up | This study |
| SmHsp* | EF103579 | Up | This study |
All the Hsps listed are heat shock-inducible, except Hsc70, which is a cognate that is constitutively expressed.
*A small Hsp of unknown size.
One of the small Hsps (clone ScD-7H7, GenBank accession no. EF103577) is a full-length clone of 1,123 bp, with a 665-bp ORF coding a 25-kDa protein with highest similarity to Drosophila Hsp27. The second small Hsp (clone ScD-9D11, GenBank accession no.EF103578) was compiled from three different clones. The full-length sequence is 851 bp, with a 483-bp ORF coding an 18-kDa protein that most closely resembles Drosophila Hsp23. Finally, a partial 431-bp clone (clone ScD-7H10, GenBank accession no. EF103579) includes the highly conserved α-crystallin domain and a portion of the more variable region 3′ of the conserved domain. All three genes show high similarity to previously described S. crassipalpis Hsp23 within the α-crystallin domain but are considerably less similar in the regions on either side. All three new small Hsps were up-regulated during diapause (Fig. 1). A fourth member of the small Hsp family, isolated while screening a previously constructed, diapause-enriched cDNA library (19) with the original Hsp23 clone as a probe, is a 1,101-bp clone (GenBank accession no. AF156162) that is possibly a pseudogene of Hsp23. Although this transcript has 99% identity with Hsp23 starting just downstream of the start codon, there is no upstream homology. The first possible start codon would yield a deduced protein of 13 kDa, and a range of experiments have failed to demonstrate an Hsp of this size in S. crassipalpis. This putative pseudogene was also highly expressed during diapause. Although pseudogenes are unable to encode proteins, we cannot discount the possibility that this pseudogene remains physiologically active, regulating the expression of its active homologs (21).
Fig. 1.
Developmental up-regulation of several Hsps (three small Hsps, an Hsp60 family member, and a new form of Hsp70) during pupal diapause in the flesh fly, S. crassipalpis, based on Northern blot hybridization. ND, nondiapausing pupae maintained at 20°C; D, diapausing pupae maintained for 20 days at 20°C.
Our SSH screening also identified a new Hsp family involved in diapause. Clone ScD-6A1 (GenBank accession no. EF103581) is a 580-bp clone with high similarity to a T-complex protein-1 (TCP-1) containing chaperonin of the Hsp60 family. The deduced 192-aa sequence exhibits substantial identity and similarity to TCP-1 from Drosophila (90% and 97%, respectively), as well as substantial similarity to the more distant brine shrimp, Artemia franciscana (74% identity and 85% similarity). Although expressed in nondiapausing individuals, transcripts of this gene were modestly up-regulated in diapausing pupae (Fig. 1).
Finally, our SSH library contained a partial clone of an additional member of the Hsp70 family, designated Hsp70B. The 640-bp clone ScD-5B11 (GenBank accession no. EF103580) includes the 3′ end of the ORF and extends through the polyA tail. Although the new Hsp70 family member is quite similar to the one previously described (18) within the ORF (93% similar at the nucleic acid level), it also exhibits major differences: there are 30- and 12-bp insertions within the ORF and no discernable similarities in the 3′ UTR region, except for the similarity in length (272 bp vs. 277 bp), the polyA signal sequence, and the polyA tail. Although the insertions are not identical to any of the five described Hsp70 sequences from Drosophila, similar insertions are reported in GenBank for the Japanese oak silkmoth Antheraea yamamai and two tick species (Ixodes scapularis and Dermacentor variabilis). Northern blot hybridization using high-stringency washes and clone ScD-5B11 as a probe yielded a single band that was up-regulated during diapause (Fig. 1).
Expression Pattern Throughout Diapause.
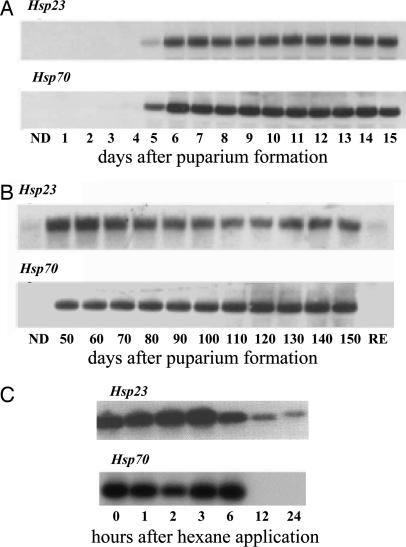
The presence of mRNAs encoding the Hsps is tightly linked to the entire dormancy period. As shown in Fig. 2A, expression of Hsp23 and Hsp70 begins 5 d after pupariation, a time that marks the onset of pupal diapause, and the mRNAs are present until adult development is initiated (Fig. 2 B and C). Approximately 60 d after the onset of diapause, the pupae enter a period of postdiapause development. During the postdiapause period, the pupae will not initiate development if they are held at low temperature, but they are fully capable of doing so if transferred to high temperatures. Under field conditions, these flies normally enter the postdiapause phase in early January, but the low temperatures that prevail during winter suppress development until soil temperatures rise in the spring (22). The Hsp mRNAs do not decline at the end of the true diapause period but persist into the postdiapause period as well (Fig. 2B). Diapause in this species can be readily terminated with the application of an organic solvent such as hexane, and when diapause is terminated in this manner, the Hsp mRNAs decline within 6–12 h (Fig. 2C). Thus, expression of the Hsps is turned on at the very beginning of diapause, and the mRNAs are present throughout diapause and postdiapause and decline within hours after the signal initiating adult development is received.
Fig. 2.
Temporal patterns of Hsp23 and Hsp70 expression in S. crassipalpis show that Hsps are turned on at the onset of pupal diapause, continue to be expressed throughout diapause and the postdiapause period, and are turned off within hours after the pupae receive a signal to resume development. (A) Expression at the onset and early phase of pupal diapause (diapause begins 5d after puparium formation). (B) During late diapause and postdiapause development (postdiapause begins ≈60 d after puparium formation). Adapted from Hayward et al. (47). (C) Rapid decline in expression of Hsp23 and Hsp70 when pupal diapause is terminated artificially by the application of hexane. Adapted from Yocum et al. (17) and Rinehart et al. (18). ND, nondiapausing pupae; RE, red eye stage of pharate adult development. The 28s was used as the control gene.
Protein Profiles Reveal Additional smHsps That Are Diapause Up-Regulated.
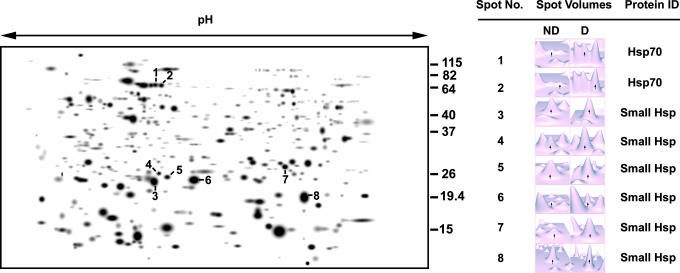
When two-dimensional electrophoresis was used to compare brain proteins in diapausing and nondiapausing pupae of S. crassipalpis, the most abundant proteins that were unique to the brains of diapausing pupae proved to be Hsps (23). As shown in Fig. 3, two of the diapause up-regulated proteins were identified as members of the Hsp70 family, and six were identified as smHsps. This approach thus confirms that mRNAs in the Hsp families are, in fact, being translated into proteins and reveals the presence of at least two additional diapause-up-regulated smHsps not previously observed.
Fig. 3.
Two-dimensional gel depicting brain proteins from diapausing pupae of S. crassipalpis. The numbered proteins, which are either unique to or more abundant in the diapausing brain, are all heat shock proteins. Numbers to the right of the gel represent kilodalton size markers. ND, nondiapause; D, diapause. Adapted from Li et al. 2007.
Hsp70 Up-Regulation in Other Species During Diapause.
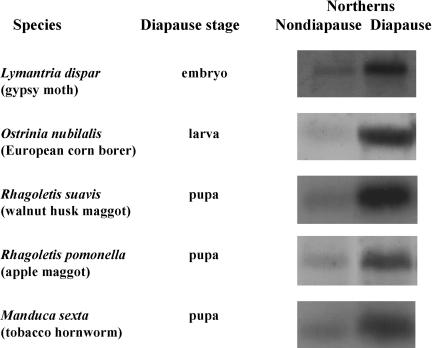
To determine how common the diapause-associated up-regulation of Hsps might be, we examined Hsp70 expression in a number of other insects, representing different orders and different stages of diapause. In all five additional species reported here, Hsp70 was up-regulated during diapause (Fig. 4). Diapause in both Rhagoletis suavis and Rhagoletis pomonella is similar to that of S. crassipalpis in that they are also dipterans that enter a facultative (environmentally programmed) diapause as pupae. Hsp70 was also diapause up-regulated in three lepidopteran species with diverse diapause programs: the obligate (genetically programmed, rather than under environmental control) embryonic diapause of Lymantria dispar, the facultative larval diapause of Ostrinia nubilalis, and the facultative pupal diapause of Manduca sexta.
Fig. 4.
Evidence that Hsp70 is up-regulated during diapause in several additional insects representing different orders and different stages of diapause. Northern blot hybridizations are based on Hsp70 clones developed for each species. L. dispar (Lepidoptera) has an obligate diapause as a late embryo (pharate first-instar larva), and the comparison is made between a chilled diapausing embryo and one that has just terminated diapause. O. nubilalis (Lepidoptera) diapauses during its last larval instar in response to short day length and low temperature; the comparison is made between nondiapausing larvae reared at long day length vs. diapausing larvae reared at short day length. R. suavis and R. pomonella (Diptera) both have a facultative pupal diapause; the comparison is made between pupae in diapause and those that have terminated diapause. M. sexta (Lepidoptera) diapauses as a pupa in response to short day length. Here, the comparison is made between a diapausing pupa reared at short day length vs. a nondiapausing pupa reared at long day length.
After our initial reports on the diapause up-regulation of Hsp23 and Hsp70 (17, 18), Hsps were found to be up-regulated during diapause in a number of additional species, including adult diapause of the Colorado potato beetle, Leptinotarsa decemlineata (24), pupal diapauses of the solitary bee, Megachile rotundata (25) and the onion maggot Delia antiqua (26), larval diapause of the rice stem borer Chilo suppressalis (27), and embryonic diapause of the silkmoth Bombyx mori (28). These published reports, together with our data on five additional species, provide evidence that Hsps are commonly up-regulated during diapause in diverse insect taxa, including Diptera, Lepidoptera, Coleoptera, and Hymenoptera and in diapauses that occur in embryonic, larval, pupal, and adult stages.
But, clearly, Hsp up-regulation is not associated with diapause in all insect species. Hsps do not appear to be up-regulated during adult diapause of Drosophila triauraria (29, 30), in larval diapause of the blow fly Lucilia sericata (31), or in adult diapause of the mosquito Culex pipiens (32). Hsp up-regulation appears to be more common in preadult diapauses than in adult (reproductive) diapauses. Although one form of Hsp70 is up-regulated during adult diapause of L. decemlineata, the up-regulation is much more modest than seen in diapauses occurring in preadult stages. It is also evident that the same Hsps are not consistently involved in diapause. Although Hsp90 is down-regulated in pupal diapause of S. crassipalpis (20), it is up-regulated in the diapauses of both D. antiqua (26) and C. suppressalis (27).
The association of Hsps with developmental arrest extends beyond insects. Hsp expression is strongly up-regulated during dormancies ranging from encystment in the brine shrimp, Artemia franciscana (33), and the quiescent stages of the freshwater sponge, Spongilla lacustris (34), to mammalian hibernation (35, 36), and small Hsps are up-regulated during dormancy in a number of plant tissues including seeds (37–39), parenchyma cells (40), and bark tissue (41). Additionally, polar organisms including larvae of the terrestrial Antarctic midge, Belgica antarctica (42), an Antarctic fish, Trematomus bernacchii, (43), and an Antarctic marine ciliate, Euplotes focardii (44), continuously express Hsps. Thus, Hsps appear to be widely used by organisms subjected to cold environments.
Hsp Function During Diapause.
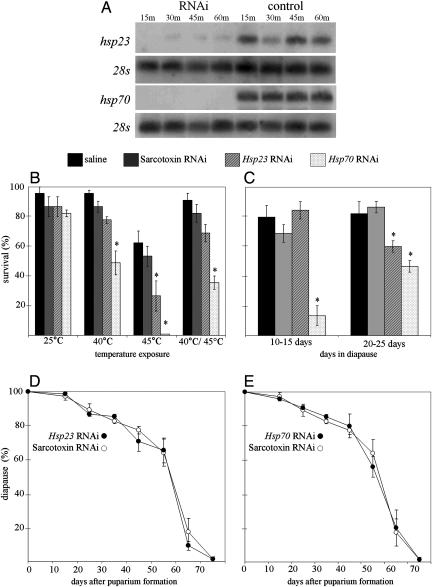
Injection of either Hsp70 or Hsp23 dsRNA into wandering third-instar larvae suppressed expression of these genes (Fig. 5A) and resulted in the predictable loss of heat tolerance in nondiapausing pupae (Fig. 5B). Although loss of heat tolerance was evident after the injection of either Hsp70 dsRNA or Hsp23 dsRNA, the effect of Hsp70 dsRNA was more pronounced. In young diapausing pupa (10–15 d in diapause), challenged with a 24-h exposure to −15°C, Hsp70 dsRNA caused a dramatic loss of cold tolerance, but Hsp23 dsRNA was ineffective (Fig. 5C). At this early stage of diapause, the cold-hardiness mechanism is not yet fully developed, but by 20–25 days, the cold hardiness of diapausing pupae is much more robust (45), and in these older pupae, RNAi directed against either Hsp70 or Hsp23 resulted in a significant loss of cold tolerance. We thus conclude that Hsp up-regulation, along with synthesis of the cryoprotectant glycerol (45) and modifications of cellular membranes (11), contribute significantly to the winter cold tolerance of diapausing flesh fly pupae. The well known role of Hsps in maintaining functional integrity of critical proteins is likely to be just as important at low temperature as it is at high temperature.
Fig. 5.
RNAi demonstrates that suppression of Hsp23 and Hsp70 expression results in a loss of both heat and cold tolerance. (A) Suppression of Hsp23 and Hsp70 expression in flesh flies that were injected as third-instar wandering larvae with 1.0 μg of dsRNA and then subjected to 45°C heat shocks of various durations. (B) Reduction in heat tolerance of flies injected with dsRNA and then exposed to 25°C (rearing temperature), 40°C for 2 h, 45°C for 1 h, or to 40°C for 2 h and then to 45°C for 1 h. Mean ± SE of three replicates of 15 individuals each. (C) Reduction of cold tolerance in diapausing pupae injected as wandering third-instar larvae with dsRNA. Survival of pupae after a 24-h exposure to −15°C was evaluated 10–15 d or 20–25 d after the onset of pupal diapause. Mean ± SE of three replicates of 15 individuals each. (D and E) Diapause incidence and duration in flies injected with Hsp23 dsRNA (D) or Hsp70 dsRNA (E) as wandering third-instar larvae. Mean ± SE of three replicates of 25 individuals each. Sarcotoxin dsRNA was used as the control in B–E. The asterisks indicates significant differences based on Tukey's test of the least-squares means.
We also tested the possibility that expression of Hsps may affect the decision to enter diapause or the duration of diapause. RNAi directed against Hsp23 (Fig. 5D) or Hsp70 (Fig. 5E) neither altered the incidence of pupal diapause nor affected how long the pupae remained in diapause.
We consider it likely that up-regulation of Hsps also provides a “back-up” mechanism guaranteeing that development will remain arrested during diapause. In most cases, expression of Hsps is incompatible with progression of development. Overexpression of Hsp70 results in a retardation of cell cycle progression (16) and a slowing of development in Drosophila melanogaster (46), and we have demonstrated that the onset of adult development is delayed when Hsps are being produced (47). We thus suspect that the presence of Hsps during diapause not only contributes to the enhancement of cold tolerance but also provides a secondary mechanism for assuring that development remains arrested.
Conclusions
We provide evidence that numerous Hsps are developmentally up-regulated during the overwintering pupal diapause of the flesh fly, S. crassipalpis. Although we previously knew that Hsp70 and Hsp23 were up-regulated during flesh fly diapause, we now show that an additional form of Hsp70, a member of the Hsp60 family, and three additional smHsps are diapause up-regulated, and a proteomics study suggests that at least six smHsps are diapause up-regulated. The fact that Hsps are up-regulated not only in diapausing flesh fly pupae but also during diapause in a variety of insect species representing different orders and different diapause stages suggests that Hsps are key players in the overwintering response of many insects. The use of RNAi to suppress expression of Hsp23 and Hsp70 demonstrates that suppression of these genes does not alter the decision to enter diapause nor does it affect the duration of the diapause, but it does have a profound effect in reducing cold tolerance of the diapausing pupae.
In several important ways, the Hsp response we note during diapause differs significantly from the typical stress response that has been well documented for Hsps. (i) The Hsps are developmentally up-regulated during diapause rather than being induced by environmental stress. (ii) The up-regulation persists for many months, presumably 9–10 months under field conditions rather than for the short intervals that characterize the stress response. (iii) In the stress response, most other genes are down-regulated when the Hsps are up-regulated, but, in diapause, the Hsps mRNAs are being transcribed concurrently with other transcripts. (iv) Unlike most stress responses that elicit synchronous up-regulation of the whole suite of Hsps, during diapause, Hsp70, Hsp60, and the smHsps are all up-regulated, but Hsp90 is actually down-regulated, thus suggesting an interesting dichotomy of function in certain Hsps under these two circumstances.
The wealth of Hsps that are up-regulated also raises unanswered questions about the specific contributions of each Hsp. Why are there so many smHsps? Is this redundancy, or do they each have a specific function? Most likely, the different smHsps are interacting differently with specific groups of proteins (48). The fact that RNAi directed against Hsp23 has less impact on cold tolerance than RNAi directed against Hsp70 already suggests that these two Hsps contribute to cold tolerance in slightly different ways.
The discovery that a member of the Hsp60 family, TCP-1, is up-regulated during flesh fly diapause is intriguing because the TCP-1 protein acts as a subunit of cytosolic chaperonin-containing TCP-1 (CCT), a large complex that facilitates the proper folding of cytoskeleton proteins such as tubulin, actin, and centractin (49–51). Several direct links have been made between TCP-1 and cold survival. In yeast, TCP-1 is up-regulated by cold shock (52), and a cold-sensitive mutant was reported to result from mutation of TCP-1 (53). More recently, the up-regulation of TCP-1 has been associated with cold hardiness in the onion maggot, D. antiqua (54), and has also been implicated in diapause of the brine shrimp, A. franciscana, where it is found in association with microtubules in undeveloped cysts (55). A link between microtubule stability and cold survival is noted in several poikilotherms adapted to cold environments (56–58), and a range of cytoskeletal adjustments are associated with insect diapause (25, 59–61). Our data, showing the up-regulation of a microtubule-specific chaperonin during diapause, further highlight the importance of cytoskeletal stability during diapause and low-temperature survival.
Cold hardiness is a component of the diapause program in the flesh fly (62). Pupae that enter diapause are considerably more tolerant of low temperatures than their nondiapausing counterparts, and the major biochemical adjustments previously recognized as contributing to this cold hardiness include accumulation of the cryoprotectant glycerol (45) and modifications of fatty acids in the cellular membrane (11). To these, we now argue for the addition of Hsps as major contributors to cold tolerance in diapausing fly pupae and overwintering stages of other insects.
Materials and Methods
Fly Rearing.
Flies were obtained from an established laboratory colony of the flesh fly, S. crassipalpis Macquart, reared under nondiapausing conditions of long day length (15:9; light/dark) at 25°C. Diapausing pupae were obtained by raising adults of the parental generation at short day length (12:12; light/dark) at 25°C and larvae at 12:12 light/dark at 20°C, as described (63). When direct comparisons were made between nondiapausing and diapausing flies, both groups were raised as larvae at 20°C while maintaining their respective light regimes. To artificially terminate diapause, the operculum of the puparium was removed, and 5 μl of hexane was applied directly to the pupal head (64).
Suppressive Subtractive Hybridization.
Clones of most newly identified Hsps reported in this study were obtained by suppressive subtractive hybridization (SSH), subtracting cDNA from nondiapausing pupae (5 d after pupariation, the developmental stage equivalent to diapause) from that of pupae that had been in diapause for 20 d, as described for the mosquito Culex pipiens (61). Briefly, RNA pools from 20 individuals were created by using TRIzol Reagent (Invitrogen, Carlsbad, CA), cDNA was synthesized by using the BD SMART PCR cDNA Synthesis kit (BD Biosciences, Franklin Lakes, NJ), and the subtraction was achieved by using a BD PCR-Select cDNA Subtraction kit (BD Biosciences). A TOPO TA Cloning kit (Invitrogen) was used for cloning, and colonies were randomly selected for sequencing.
Northern Blot Hybridization.
Samples of RNA used in Northern blot hybridization consisted of 20 μg of total RNA isolated from three pupae by using TRIzol reagent following standard protocol. Samples were heat-denatured before electrophoretic separation on a 1.5% agarose, 0.41M formaldehyde gel, transferred to a charged nylon membrane (Osmonics, Minnetonka, MN) by using a Turbo-Blotter (Schleicher & Schuell, Florham Park, NJ), and cross-linked by UV irradiation. Clones of interest were digoxigenin (DIG)-labeled by using DIG-high prime solution (Roche Applied Science, Indianapolis, IN). Full-length clones were used for all Northern blots except for those confirming gene suppression by RNAi. For the RNAi Northern blots, probes were made from the portion of the clone that was not used as a template for dsRNA synthesis (bases 1–570 for Hsp23 and bases 1–743 for Hsp70). In all instances, the resulting probes were then used in Northern blot hybridization using the Dig High Prime DNA Labeling and Detection Starter Kit II (Roche Applied Science) following standard protocol. BioMax Chemiluminescence film (Kodak, Rochester, NY) was then exposed to the blot for signal detection. Equal loading of samples was confirmed by alkaline stripping the membrane by using 0.2 M NaOH, 0.1% SDS, followed by reprobing with a 28s rRNA probe. All Northern blots were run in triplicate.
Hsp70 from Other Insects.
A partial clone of Hsp70 was obtained from the following insects by using PCR primers designed from conserved insect sequences following established protocol (32). The resulting PCR products were subsequently cloned and sequenced. Partial clones were developed for the walnut husk maggot R. suavis (GenBank accession no. EF103585), the gypsy moth, Lymantria dispar (GenBank accession no. EF103582), the European corn borer, Ostrinia nubilalis (GenBank accession no. EF103583), and the tobacco hornworm, M. sexta (GenBank accession no. AF533366). A partial clone was also developed for the apple maggot Rhagoletis pommonella, followed by 3′ and 5′ RACE to develop a full-length clone (GenBank accession no. EF103584).
These clones were subsequently used as probes in Northern blot hybridization to characterize Hsp70 expression during diapause. For R. pommonella and R. suavis, both of which enter a facultative pupal diapause (65), comparisons were made between pupae diapausing at 4°C with those in which diapause was broken with a 3-d exposure to 25°C. L. dispar enters an obligate diapause, although, in this case, as a pharate first-instar larva still encased within the chorion of the egg (66, 67). For L. dispar, the comparison was made between diapausing individuals held at 4°C and those in which diapause was broken by a 3-d exposure to 25°C. In contrast, O. nubilalis exhibits a facultative, fifth (final) larval diapause, induced by short day length and low temperature (68). M. sexta enters a facultative pupal diapause, programmed by short day length (69). For both O. nubilalis and M. sexta, comparisons were made between diapausing pupa and their nondiapausing counterparts, all held at 20°C. All Northern blots were done in triplicate, with equal loading determined by ethidium bromide staining of ribosomal RNA.
dsRNA Synthesis and Injection.
Our dsRNA synthesis protocol was adapted from that of Kennerdell and Carthew (70), by using a PCR template method of production. Primers specific to Hsp23 and Hsp70 from S. crassipalpis were constructed with the addition of a 27 nucleotide T7 sequence to the 5′ end of each primer. The primers for Hsp23 were designed to amplify bases 570 through 1016 of the Hsp23 sequence (GenBank accession no. U96099), resulting in a 446bp fragment that included the ORF 3′ of the conserved α-crystalline domain and most of the 3′ UTR. The primers for Hsp70 were designed to amplify bases 797 through 1225 of the partial Hsp70 sequence (GenBank accession no. AF107338). As a control, we also developed dsRNA for sarcotoxin, a gene involved in immune function of S. crassipalpis (71). The primers for sarcotoxin were designed to amplify bases 1–546 of the partial sequence (GenBank accession no. AY130768). The Supermix (Invitrogen)-based PCR used the respective clones in pCR 2.1 plasmids (Invitrogen) as a template with 10 cycles by using a 40°C annealing temperature, followed by 35 cycles annealing at 55°C. After amplification, the resulting product was phenol–chloroform extracted, ethanol precipitated, and reconstituted in Tris buffer. RNA synthesis was next conducted by using T7 RNA polymerase (Promega, Madison, WI) on 1 μg of PCR product following standard protocol. After DNase treatment to remove template, the reaction was again phenol–chloroform extracted and ethanol precipitated before storage at −70°C under ethanol.
Our dsRNA injection protocol was adapted from Nishikawa and Natori (72), by using third-instar wandering larvae that had already purged their guts. dsRNA was dissolved in insect saline (130 mM NaCl, 5 mM KCl, and 1 mM CaCl2) to a concentration of 0.2 μg/μl, and 5 μl was injected into each larva by using a pulled-glass capillary needle. Larvae were cold-anesthetized on ice for 30 min before and after injection.
Temperature Exposure.
For the high- and low-temperature exposures, 15 pupae were placed in thin-walled, 13 × 100-mm cotton-plugged Pyrex test tubes that were placed in a Lauda model RM20 glycerol bath set at the desired temperature. Survival of nondiapausing pupae at high temperature was assessed by monitoring successful adult eclosion. Survival of diapausing pupae at low temperature was assessed by monitoring the status of the pupae 1 week later; healthy pupae remained white, whereas unhealthy pupae were dark. All treatments were performed in triplicate, and data were analyzed by two-way ANOVA with replication, followed by Tukey's test of the least-squares means, with P < 0.05 considered to be statistically significant.
Diapause Incidence and Duration.
Fifteen days after pupariation, the operculum was removed from the puparium of flies reared in diapause-inducing conditions (12:12 light/dark, 20°C), and the diapause status of the pupae was noted by determining whether adult development had been initiated (73). To determine diapause duration, pupae were subsequently inspected at 10-d intervals to note the time of diapause termination, as evidenced by the migration of antennal discs and the appearance of red pigmentation in the eyes. All assessments were done in triplicate.
Acknowledgments
We thank Tracy Craig, Bryan Cairns, Jakub Godlweski, and Katie Yoders for assistance in these experiments and Professors Richard E. Lee, Jr., (Miami University, Oxford, OH) and Thomas H. MacRae (Dalhousie University, Halifax, NS, Canada) for helpful comments on the manuscript. This work was supported by U.S. Department of Agriculture–National Research Initiative Grant 2005-03407), National Science Foundation Grant IOB-0416720, and National Institutes of Health Grant R01 AI058279.
Abbreviations
- Hsp
heat shock protein
- CCT
cytosolic chaperonin containing T-complex
- TCP-1
T-complex protein-1
- SSH
suppressive subtractive hybridization.
Footnotes
References
- 1.Tauber MJ, Tauber CA, Masaki S. Seasonal Adaptations of Insects. Oxford: Oxford Univ Press; 1986. [Google Scholar]
- 2.Danks HV. Insect Dormancy: An Ecological Perspective. Ottawa, Canada: Biological Survey; 1987. [Google Scholar]
- 3.Denlinger DL, Yocum GD, Rinehart JP. In: Comprehensive Insect Molecular Science. Gilbert LI, Iatrou K, Gill S, editors. Vol 3. Amsterdam: Elsevier; 2005. pp. 615–640. [Google Scholar]
- 4.Denlinger DL. Annu Rev Entomol. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- 5.Lee RE. In: Insects at Low Temperature. Lee RE, Denlinger DL, editors. New York: Chapman & Hall; 1991. pp. 17–46. [Google Scholar]
- 6.Crowe JH, Crowe LM, Carpenter JF, Rudolph AS, Wistrom CA, Spargo BJ, Anchordoguy TJ. Biochem Biophys Acta. 1988;947:367–384. doi: 10.1016/0304-4157(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 7.DeVries A. Science. 1971;172:1152–1155. doi: 10.1126/science.172.3988.1152. [DOI] [PubMed] [Google Scholar]
- 8.Duman JG, Xu L, Neven LG, Tursman D, Wu DW. In: Insects at Low Temperature. Lee RE, Denlinger DL, editors. New York: Chapman & Hall; 1991. pp. 94–127. [Google Scholar]
- 9.Koštál V, Berková P, Šimek P. Comp Biochem Physiol B. 2003;135:407–419. doi: 10.1016/s1096-4959(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 10.Overgaard J, Sørenson JG, Petersen SO, Loeschcke V, Holmstrup M. J Insect Physiol. 2005;51:1173–1182. doi: 10.1016/j.jinsphys.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Michaud MR, Denlinger DL. J Insect Physiol. 2006;52:1073–1082. doi: 10.1016/j.jinsphys.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Lee RE, Damodaran K, Yi S-X, Lorigan GA. Cryobiology. 2006;52:459–463. doi: 10.1016/j.cryobiol.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Parsell DA, Lindquist S. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 14.Feder ME, Hofmann GE. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, MacRae TH. FEBS J. 2005;272:2613–2627. doi: 10.1111/j.1742-4658.2005.04708.x. [DOI] [PubMed] [Google Scholar]
- 16.Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S. Genes Dev. 1992;6:1402–1413. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- 17.Yocum GD, Joplin KH, Denlinger DL. Insect Biochem Mol Biol. 1998;28:677–682. doi: 10.1016/s0965-1748(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 18.Rinehart JP, Yocum GD, Denlinger DL. Insect Biochem Mol Biol. 2000;30:515–521. doi: 10.1016/s0965-1748(00)00021-7. [DOI] [PubMed] [Google Scholar]
- 19.Flannagan RD, Tammariello SP, Joplin KH, Cirka-Ireland RA, Yocum GD, Denlinger GD. Proc Natl Acad Sci USA. 1998;95:5616–5620. doi: 10.1073/pnas.95.10.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinehart JP, Denlinger DL. Insect Mol Biol. 2000;9:641–645. doi: 10.1046/j.1365-2583.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 21.Korneev SA, Park JH, O'Shea M. J Neurosci. 1999;19:7711–7720. doi: 10.1523/JNEUROSCI.19-18-07711.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denlinger DL. Ann Entomol Soc Am. 1972;65:410–414. [Google Scholar]
- 23.Li AQ, Popova-Butler A, Dean DH, Denlinger DL. J Insect Physiol. 2007;53:385–391. doi: 10.1016/j.jinsphys.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Yocum GD. J Insect Physiol. 2001;47:1139–1145. doi: 10.1016/s0022-1910(01)00095-6. [DOI] [PubMed] [Google Scholar]
- 25.Yocum GD, Kemp WP, Bosch J, Knoblett JN. J Insect Physiol. 2005;51:621–629. doi: 10.1016/j.jinsphys.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Chen B, Kayukawa T, Monteiro A, Ishikawa Y. Insect Mol Biol. 2005;14:697–702. doi: 10.1111/j.1365-2583.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 27.Sonoda S, Fukumoto K, Izumi Y, Yoshida H, Tsumuki H. Arch Insect Biochem Physiol. 2006;63:36–47. doi: 10.1002/arch.20138. [DOI] [PubMed] [Google Scholar]
- 28.Hwang J-S, Go H-J, Goo T-W, Yun E-Y, Choi K-H, Seong S-I, Lee S-M, Kim I, Kang S-W. Arch Insect Biochem Physiol. 2005;59:197–201. doi: 10.1002/arch.20057. [DOI] [PubMed] [Google Scholar]
- 29.Goto SG, Yoshida KM, Kimura MT. J Insect Physiol. 1998;44:1009–1015. doi: 10.1016/s0022-1910(97)00143-1. [DOI] [PubMed] [Google Scholar]
- 30.Goto SG, Kimura MT. Gene. 2004;326:117–122. doi: 10.1016/j.gene.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Tachibana SI, Numata H, Goto SG. J Insect Physiol. 2005;51:641–647. doi: 10.1016/j.jinsphys.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Rinehart JP, Robich RM, Denlinger DL. J Med Entomol. 2006;43:713–722. doi: 10.1603/0022-2585(2006)43[713:ECADTI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.MacRae TH. Semin Cell Dev Biol. 2003;14:251–258. doi: 10.1016/j.semcdb.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Schill RO, Pfannkuchen M, Fritz G, Köhler H-R, Brümmer F. J Exp Zool. 2006;305:A449–A457. doi: 10.1002/jez.a.281. [DOI] [PubMed] [Google Scholar]
- 35.Eddy SF, McNally JD, Storey KB. Arch Biochem Biophys. 2005;435:103–111. doi: 10.1016/j.abb.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Carey HV, Sills NS, Gorham DA. Am Zool. 1999;39:825–835. [Google Scholar]
- 37.Coca MA, Almonguera C, Jordano J. Plant Mol Biol. 1994;25:479–492. doi: 10.1007/BF00043876. [DOI] [PubMed] [Google Scholar]
- 38.DeRocher AE, Vierling E. Plant J. 1994;5:93–102. [Google Scholar]
- 39.Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E. Plant Physiol. 1996;112:747–757. doi: 10.1104/pp.112.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ukaji N, Kuwabara C, Takezawa D, Arakawa K, Yoshida S, Fujikawa S. Plant Physiol. 1999;120:481–489. doi: 10.1104/pp.120.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bassett CL, Wisniewski ME, Artlip TS, Norelli JL, Renaut J, Farrell RE. J Am Soc Hortic Sci. 2006;131:551–563. [Google Scholar]
- 42.Rinehart JP, Hayward SAL, Elnitsky MA, Sandro LH, Lee RE. Proc Natl Acad Sci USA. 2006;103:14223–14227. doi: 10.1073/pnas.0606840103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckley BA, Place SP, Hofmann G. J Exp Biol. 2004;207:3649–3656. doi: 10.1242/jeb.01219. [DOI] [PubMed] [Google Scholar]
- 44.La Terza A, Papa G, Miceli C, Alimenti C, Luporini P. Mol Ecol. 2001;l0:1061–1067. doi: 10.1046/j.1365-294x.2001.01242.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee RE, Chen C-P, Meacham MH, Denlinger DL. J Insect Physiol. 1987;33:587–592. [Google Scholar]
- 46.Krebs RA, Feder ME. Cell Stress Chaperones. 1997;2:60–71. doi: 10.1379/1466-1268(1997)002<0060:dcohoi>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayward SAL, Pavlides SC, Tammariello SP, Rinehart JP, Denlinger DL. J Insect Physiol. 2005;51:631–640. doi: 10.1016/j.jinsphys.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, MacRae TH. Cell Mol Life Sci. 2005;62:2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartl FU, Hayer-Hartl M. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 50.Bertrand S, Barthelemy I, Oliva MA, Carrascosa JL, Andreu JM, Valpuesta JM. J Mol Biol. 2005;346:319–330. doi: 10.1016/j.jmb.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 51.Liang P, MacRae TH. J Cell Sci. 1997;110:1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- 52.Somer L, Shmulman O, Dror T, Hashmueli S, Kashi Y. Cell Stress Chaperones. 2002;7:47–54. doi: 10.1379/1466-1268(2002)007<0047:teccia>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Sullivan DS, Huffaker TC. Proc Natl Acad Sci USA. 1994;91:9111–9115. doi: 10.1073/pnas.91.19.9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kayukawa T, Chen B, Miyazaki S, Itoyama K, Shinoda T, Ishikawa Y. Cell Stress Chaperones. 2005;10:204–210. doi: 10.1379/CSC-106R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Connell PA, Pinto DM, Chisholm KA, MacRae TH. Biochim Biophys Acta. 2006;1764:920–928. doi: 10.1016/j.bbapap.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Detrich HW, Johnson KA, Marchese-Ragona SP. Biochemistry. 1989;28:10085–10093. doi: 10.1021/bi00452a031. [DOI] [PubMed] [Google Scholar]
- 57.Pucciarelli S, Ballarini P, Miceli C. Cell Motil Cytoskeleton. 1997;38:329–340. doi: 10.1002/(SICI)1097-0169(1997)38:4<329::AID-CM3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 58.Pucciarelli S, Miceli C. Extremophiles. 2002;6:385–389. doi: 10.1007/s00792-002-0268-5. [DOI] [PubMed] [Google Scholar]
- 59.Lee K-Y, Hiremath S, Denlinger DL. J Insect Physiol. 1998;44:221–226. doi: 10.1016/s0022-1910(97)00173-x. [DOI] [PubMed] [Google Scholar]
- 60.Kim M, Robich RM, Rinehart JP, Denlinger DL. J Insect Physiol. 2006;52:1226–1233. doi: 10.1016/j.jinsphys.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robich RM, Rinehart JP, Kitchen LJ, Denlinger DL. J Insect Physiol. 2007;53:235–245. doi: 10.1016/j.jinsphys.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adedokun TA, Denlinger DL. Physiol Entomol. 1984;9:361–364. [Google Scholar]
- 63.Denlinger DL. Biol Bull, Woods Hole. 1972;142:11–24. [Google Scholar]
- 64.Denlinger DL, Campbell JJ, Bradfield JY. Physiol Entomol. 1980;5:7–15. [Google Scholar]
- 65.Prokopy RJ. Can Entomol. 1968;100:318–329. [Google Scholar]
- 66.Masaki S. Jpn J Appl Zool. 1956;21:148–157. [Google Scholar]
- 67.Leonard DE. J Econ Entomol. 1968;61:596–598. [Google Scholar]
- 68.Beck SD. J Insect Physiol. 1982;28:273–277. [Google Scholar]
- 69.Bell RA, Rasul CG, Joachim FG. J Insect Physiol. 1975;21:1471–1480. [Google Scholar]
- 70.Kennerdell JR, Carthew RW. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- 71.Rinehart JP, Diakoff SJ, Denlinger DL. Eur J Entomol. 2003;100:251–254. [Google Scholar]
- 72.Nishikawa T, Natori S. Eur J Biochem. 2001;268:5295–5299. doi: 10.1046/j.0014-2956.2001.02461.x. [DOI] [PubMed] [Google Scholar]
- 73.Fraenkel G, Hsiao C. J Insect Physiol. 1968;14:689–705. [Google Scholar]