Abstract
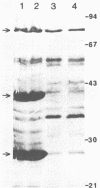
Comparative analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of culture supernatants of a virulent Pseudomonas solanacearum strain and of a spontaneous avirulent mutant derived from it was performed. The results show that the levels of two major polypeptides with molecular masses of 43 and 25 kilodaltons (kDa) were markedly reduced in the spent culture medium of the avirulent mutant. In addition, enzyme assays showed that the level of carboxymethyl cellulase (endoglucanase) activity in the culture supernatants of the avirulent mutant was reduced over 25-fold, whereas polygalacturonase activities in both strains were nearly identical. Purification of the endoglucanase from the spent culture medium of the virulent P. solanacearum strain by adsorption to phosphocellulose, salt elution, and gel-filtration chromatography yielded a >95% pure preparation of the 43-kDa polypeptide. The kinetic and enzymatic properties of the purified endoglucanase were subsequently analyzed. Antibody prepared against the purified 43-kDa endoglucanase was used to demonstrate its production by several strains of P. solanacearum races 1 and 2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Keen N. T., Williams P. H., Walker J. C. Protease of Pseudomonas lachrymans in relation to cucumber angular leaf spot. Phytopathology. 1967 Mar;57(3):263–271. [PubMed] [Google Scholar]
- Kelman A., Hruschka J. The role of motility and aerotaxis in the selective increase of avirulent bacteria in still broth cultures of Pseudomonas solanacearum. J Gen Microbiol. 1973 May;76(1):177–188. doi: 10.1099/00221287-76-1-177. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]