Abstract
During development of the nervous system, the tip of a growing axon, the growth cone (GC), must respond accurately to stimuli that direct its growth. This axonal navigation depends on extracellular concentration gradients of numerous guidance cues, including GABA. GCs can detect even weak directional signals, yet the mechanisms underlying this sensitivity remain unclear. Past studies in other eukaryotic chemotactic systems have pointed to the role of the spatial reorganization of the transduction pathway in their sensitive response. Here we have developed a single-molecule assay to observe individual GABAA receptors (GABAARs) in the plasma membrane of nerve GCs subjected to directional stimuli. We report that in the presence of an external GABA gradient GABAARs redistribute asymmetrically across the GC toward the gradient source. Single-particle tracking of GABAARs shows that the redistribution results from transient interactions between the receptors and the microtubules. Moreover, the relocalization is accompanied by an enhancement in the asymmetry of intracellular calcium concentration. Altogether, our results reveal a microtubule-dependent polarized reorganization of chemoreceptors at the cell surface and suggest that this polarization serves as an amplification step in GABA gradient sensing by nerve GCs.
Keywords: axonal guidance, chemotaxis, single molecule, polarity
Chemotactic response to directional stimulations is a key element for the construction of the nervous system (1–3). Axonal navigation in external gradients has been described for different guidance signals, including neurotrophins (4), netrins (5), semaphorins (6), homeoproteins (7), and neurotransmitters (8). The end tip of the axon, the growth cone (GC), is a highly efficient gradient sensor, which can detect concentration difference across its spatial extent below a couple of percents (5, 9). The guidance mechanisms depend on complex signaling cascades leading to rearrangement of the cytoskeleton and, ultimately, motility of the GC (10). Over the last decade, numerous studies have focused on deciphering these cascades and identifying the role of various signaling molecules (1–3). In contrast, the spatiotemporal organization of the transduction machinery has been the subject of few investigations. This aspect is, however, essential for the sensitive response to gradient of external cues. Past studies on chemotaxis in nonneuronal cells, such as neutrophils and Dictyostelyum discoideum amoebas, have indeed provided models explaining the conversion of a shallow chemoattractant gradient into a steeper internal gradient (11) based on the generation of an asymmetric localization of molecules in the signaling pathway (12). In neutrophils and amoebas this asymmetric distribution is a critical step in the hierarchical sequence of events that follow signal sensing and lead to cell motility (11). Understanding the mechanisms by which a polarized distribution of signaling molecules is generated and the implication of diffusive or cytoskeleton-dependent active transport in this process is thus essential in accounting for the modulation of the cell response to external directional stimuli.
In GCs little is known about a spatial redistribution of signaling molecules during guidance. However, the asymmetric localization of lipid rafts and raft-associated tyrosine kinase B receptors after exposure to a brain-derived neurotrophic factor gradient has been reported (13). Given the alleged role of rafts as signaling platforms, the membrane reorganization could serve to locally enhance the sensing efficiency at the GC leading edge by regulating the number of active receptors; however, the timing and the mechanism of the reorganization are unknown. Two groups have also recently shown that asymmetric localization and translation of β-actin mRNAs plays an essential role in GC turning (14, 15). These findings further emphasize the importance of elucidating the spatial organization in the GC during guidance.
Here we investigated the membrane dynamics of chemoreceptors in the GC during stimulation by guidance cues. We developed a single-molecule assay in which the spatiotemporal dynamics of the distribution of individual GABAARs is monitored in response to a GABA gradient. The role of GABA as chemoattractant, like other neurotransmitters such as acetylcholine (Ach) and glutamate (8), has been previously documented (16). Our assay takes advantage of the brightness and photostability of quantum dots (QDs), which are used to fluorescently tag GABAARs and to track them at the single-nanoparticle level over long durations (tens of minutes), with good signal-to-noise ratio (≈30) and with high localization accuracy (≈10 nm) (17, 18). Moreover, the ability to carry out parallel acquisition of multiple individual QD-tagged molecules is critical to quantitatively investigate changes in their spatial distribution in response to an external signal. This cannot be achieved with organic fluorophores, for which photobleaching limits single-molecule recordings to a couple of seconds, or with micrometer-sized latex beads.
We report that the GABAA receptors (GABAARs) redistribute asymmetrically across the GC toward the gradient source in a microtubule (MT)- and calcium-dependent manner. Analysis of the trajectories of individual receptors enabled the characterization of a “conveyor belt” motion in which receptors alternated between MT-dependent directed movements and free diffusion in the membrane. These reversible interactions between receptors and MTs provide a positive-feedback loop that accounts for the asymmetric redistribution of receptors in the membrane. Furthermore, this GABA-specific redistribution leads to enhancement in the asymmetry of the intracellular calcium concentration, a central messenger in the transduction of the guidance signal into motile response. Our observations suggest that reorganization of GABAARs could serve for the amplification of GABA gradient sensing by nerve GCs.
Results
Redistribution of the Receptors Toward the Source of GABA.
We first used a standard turning assay (8) to test the role of a GABA gradient in our cultured spinal cord neurons (3–6 days in vitro). A pipette placed 100 μm away at a 45° angle from the GC ejected droplets (≈1 picoliter) of 10 mM GABA at 2 Hz. At the GC this created a permanent gradient with an average concentration of 10 μM and a slope of ≈0.1 μM/μm. Under this stimulation, GCs started to turn toward the GABA source after an average of 33 min (SD = 8 min; n = 17 cells). After 1 h the average deviation angle was 17.1° (SD = 4.9°) [supporting information (SI) Fig. 6]. These observations are in agreement with previous reports identifying GABA as a chemoattractant (16).
We next combined a GC guidance assay with single QD imaging (17, 18) to study the lateral dynamics of individual GABAARs when placed in a GABA gradient (Fig. 1A and SI Text). We labeled the γ2 subunits, known to be present in functional receptors (19), with biotinylated antibodies and streptavidin-coated QDs such that 10–30 individual QDs (identified by their fluorescence intermittency) could be detected simultaneously in a GC (17). No internalization of the tagged receptors was observed, even after 40 min. Upon application of the external gradient, we observed that, before GC turning, GABAARs tended to accumulate on the side of the GC facing the gradient (see SI Fig. 7). However, dynamic fluctuations in the morphology and orientation of the fast-moving GCs precluded a quantitative analysis of the receptor distribution. Consequently, we focused on pausing GCs whose morphology and orientation remained unchanged during the experiment (≈20 min) and that were exposed to a gradient of constant direction and amplitude. In the following, the pipette was placed along the y-axis, at 90° from the GC axis (x-axis), defined as the axis of the parent axon (Fig. 1A). We defined a proximal region on the side of the GC facing the source of the gradient and a distal region by dividing the GC along the x-axis.
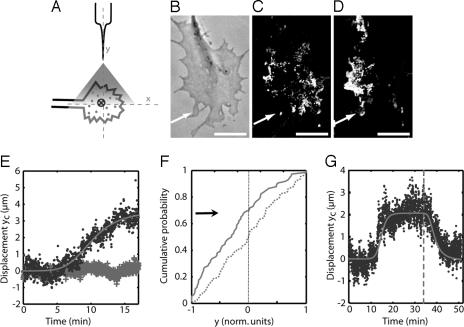
Fig. 1.
Receptor redistribution under a GABA gradient. (A) Principle of the measurement of the distribution of receptors in the presence of an external gradient of GABA: individual QD-tagged receptors are detected by their fluorescence, and their center of gravity (cross) is determined. (B–D) Effect of a GABA gradient on the distribution of GABAARs in the GC. The arrow indicates the direction of the source. (B) Transmission image. (C and D) Images corresponding for each pixel to the maximum over the sequence of images recorded during the first 10 min of simulation (C) and during the next 10 min (D). These images provide a visualization of the membrane area explored by all of the receptors during each period. (Scale bar: 10 μm.) (E) Displacement yc(t) (averaged over nine GCs) of the center of gravity along the y-axis in the presence (dots) or the absence (crosses) of external GABA gradient. The line, used as a guide for the eye, is an adjustment with a saturating power law Atm/(Bm + tm) (m = 4.9, B = 10.3 min). (F) Cumulative probability of the spatial distribution of receptors along the y-axis before (dashed line) and after (plain line) application of a gradient (P < 10−6, Kolmogorov–Smirnov test). The y coordinate is expressed in normalized units where −1 and +1 correspond to the extremities of the proximal and distal region, respectively. (G) Reversibility of the redistribution in the membrane (in one GC). After the gradient was removed (at t = 34 min, dashed line) the receptors returned to a symmetric distribution.
We acquired time-lapse sequences of fluorescence images of GABAARs (one 75-ms image per second for ≈20 min). We observed that GABAARs kept diffusing rapidly in the GC during the full sequence. In addition, their distribution appeared progressively biased toward the gradient source (80% of the GCs; n = 11) (Fig. 1 B–D). To quantify this observation, we determined in each image the position yc along the y-axis of the center of gravity of the tagged receptors (Fig. 1E). yc was close to zero during the first ≈5 min, indicating that the GABAARs remained symmetrically distributed (with respect to the x-axis) in the GC. Afterward, yc increased and subsequently saturated, with a half-time Tr = 10.3 min (SD = 1.8 min; n = 9), toward a value of ≈3.5 μm (≈25% of the full dimension of the GCs along the y-axis) (Fig. 1E). Thus, the distribution of receptors became asymmetric, with approximately two-thirds of the receptors in the proximal region (Fig. 1F). Furthermore, the initial symmetric distribution was reestablished within a couple of minutes after removing the external gradient (66% of observed GCs; n = 3) (Fig. 1G).
We checked that the asymmetric redistribution observed by sampling the population of GABAARs with single QD imaging did not result from our labeling procedure using divalent primary antibodies. First, an external GABA gradient was applied to unlabeled GC. After 30 min cells were rapidly fixed and immunostained with a primary antibody against GABAARs, followed by fluorescent secondary antibodies. The ratio r of the fluorescence signal between the proximal and distal region was 1.9 ± 0.1 (n = 7), significantly larger (P < 10−4, Kolmogorov–Smirnov test) than the value in control conditions (SI Fig. 8). This result unambiguously demonstrated that the redistribution observed with QDs was not an artifact of our experimental approach and reflected a physiological situation. Moreover, the redistribution of GABAARs was not altered when receptors were bound to the primary antibody before the gradient application. After a 30-min gradient, the cells were fixed and labeled with a secondary antibody, leading to r = 2.2 ± 0.1 (P < 0.01; n = 4). In both situations, the redistribution amplitude (indicated by the value of r) was consistent with the value of ≈2 found by using single QD measurements (Fig. 1F).
Specificity of the Redistribution.
To investigate the mechanisms underlying the asymmetric redistribution of GABAARs, we first measured the lateral diffusion of neural cell adhesion molecule (N-CAM), an unrelated membrane-bound protein, and found that, in a GABA gradient, N-CAM molecules remained symmetrically localized (SI Fig. 9). This rules out that GABAAR redistribution results from of a membrane flow and argues in favor of an active and specific mechanism.
We subsequently tested the specificity of the redistribution for GABA signals. When GABA was replaced with Ach, another chemoattractant (8), it did not lead to an asymmetric distribution of GABAARs in the GCs (Fig. 2A, crosses). The receptor relocalization was also abolished in GCs subjected to both a GABA gradient and a gabazine bath (a specific antagonist of GABAARs) (Fig. 2B). These results show that the redistribution of GABA receptors is specific to the pathway and cannot be induced by nonspecific reorganization of the cytoskeleton. However, when GCs were placed in a GABA bath (10 μM), to activate homogeneously the receptors, and submitted to an Ach gradient, we observed a redistribution of GABAARs (≈80% of GCs; n = 9) (Fig. 2A). This result indicates that relocalization of GABAARs involves components of the steering machinery, such as the cytoskeleton, which are common to different guidance pathways, but that receptor activation is required for their redistribution.
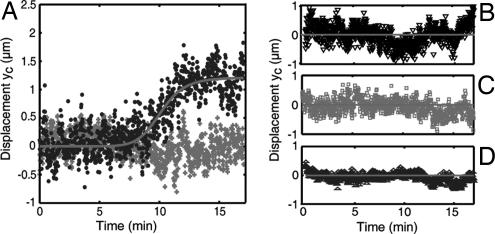
Fig. 2.
Pharmacological analysis of the receptor redistributor. (A) Displacement yc(t) in the presence of an Ach gradient (crosses, averaged on 10 GCs) and in the presence of an additional bath of 10 μM GABA (dots, averaged for seven GCs responding to Ach). The line, used as a guide for the eye, is an adjustment with a saturating power law Atn/(Bn + tn). (B–D) Displacement yc(t) when the neurons were treated with 5 μM gabazine (B), with 1 μM nocodazole for 60 min (C), or with 30 μM BAPTA-AM for 30 min (D).
MT- and Calcium-Dependent Redistribution.
Pharmacological treatments provided additional information on the mechanisms controlling the distribution of GABAARs. We tested the role of MTs, which play a crucial role in axon growth and in directional sensing mechanisms (10, 20) and interact, at least indirectly, with GABAARs (21, 22). We found that the asymmetric redistribution of the receptors was suppressed when MTs were depolymerized with nocodazole (Fig. 2C). The asymmetric relocalization was also suppressed by application of the calcium chelator BAPTA-AM (Fig. 2D), emphasizing the role of Ca2+, a known messenger in GC gradient sensing (23) and in the signaling cascade of GABA (24). These results highlight the role of downstream components of the signaling pathway in the receptor reorganization and points to a cytoskeleton-driven redistribution of GABAARs.
Conveyor Belt Motion of the GABAARS in the Membrane.
We next probed the mechanisms responsible for their asymmetric, but reversible, redistribution of GABAARs in the membrane. In particular, we sought to determine the processes preventing the receptors from being uniformly distributed due to their rapid lateral diffusion. We used single QD tracking to analyze individual trajectories of GABAARs in continuous sequence of images and determine the parameters controlling their membrane dynamics in the absence and presence of external GABA signals (Fig. 3A).
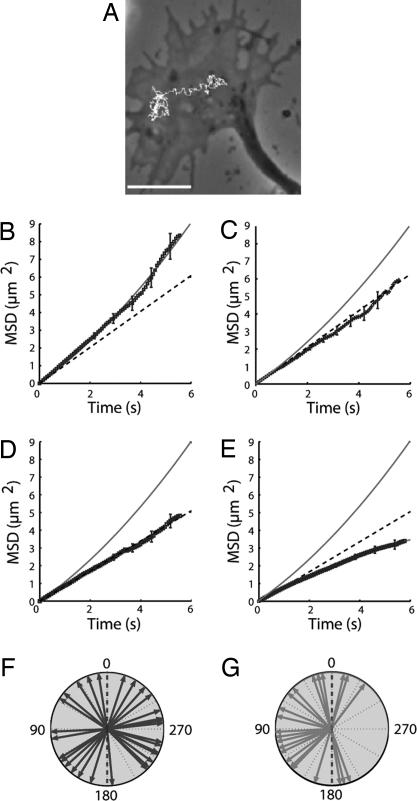
Fig. 3.
Analysis of the receptor dynamics. (A) Example of a receptor trajectory. (Scale bar: 10 μm.) (B–E) MSD (points) versus time for receptors in the C-region of GCs (B), in the P-region (averaged over 38 trajectories) (C), in the C-region after treatment with nocodazole (averaged over 103 trajectories) (D), and in the C-region after treatment with taxol (averaged over 97 trajectories) (E). The line corresponds to a quadratic curve 4Dt + v2t2 with D = 0.25 μm2·s−1 and v = 0.29 μm·s−1. The dashed lines are linear plots 4Dt with values of D given in the text. (F and G) Angular orientation of individual receptor trajectories with respect to the x-axis of the GC (dashed line) measured on three different GCs at the beginning of stimulation (n = 38) (F) and after 10 min (n = 22) (G). The arrows represent the angle θ of the displacement calculated from the first and the last points of each trajectory. The gradient source is positioned at 90°. The distributions of θ were significantly different (P < 0.01, Kolmogorov–Smirnov test).
In the absence of GABA, the mean-square displacement (MSD) of 80% of the receptor trajectories located in the MT-rich central region (C-region) of the GC fitted a curve defined by 4Dt + v2t2 (Fig. 3B). This indicated that the receptor motion consisted of Brownian diffusion (with coefficient D) associated with directed movement (with velocity v) (25). The mean value of D (Dm ± SEM) was 0.25 ± 0.01 μm2·s−1 and that of v (vm,± SEM) was 0.29 ± 0.02 μm·s−1 (n = 92; SI Fig. 10) (26). This value of vm is close to the elongation speed of MTs in cultured neurons (27), and that of Dm is compatible with a protein diffusing in the plasma membrane (17, 25). Similar values for vm and Dm were found with dye-labeling of the receptors, indicating that the GABAAR lateral dynamics is not affected by QD size or its multivalence (SI Fig. 11). In the actin-rich peripheral regions of the GC the movement of receptors was purely diffusive (Dm = 0.26 ± 0.02 μm2·s−1; n = 38) (Fig. 3C). Thus, even though it is essential for GC motility (10), the actin network is not directly involved in the lateral dynamics of GABAARs.
When neurons were incubated with 1 μM nocodazole for 1 h, directed motion was abolished in 95% of trajectories in the C-region as evidenced by the linear variation of the MSD (Fig. 3D). The movements were then purely Brownian (Dm = 0.22 ± 0.01 μm2·s−1; n = 103) (SI Fig. 12). After washing for 2 h in fresh medium, 80% of receptors recovered their directed motion with a vm = 0.27 ± 0.02 μm·s−1 (n = 35) (SI Fig. 12), similar to the vm before treatment.
The motion of the receptors is MT-dependent and could originate from their coupling to molecular motors or from interactions with growing MTs. However, when MTs were blocked in their polymerized state with taxol at 10 μg/ml for 1 h, the MSD displayed a saturating behavior (Fig. 3E). This negative curvature indicates that the directed movements were abolished and the receptor diffusion was confined or slowed. The results of the experiments with taxol thus do not favor the hypothesis of the interactions with a motor. The directed movements were more likely due to the receptors binding to MT ends, possibly by means of other molecular partners (21, 22), and being displaced during MT polymerization.
Close analysis of individual GABAAR trajectories further revealed an alternation of phases of diffusion and directed motion. To quantitatively characterize this dynamic interplay we used a speed correlation index based on analysis of the correlation between the velocities at consecutive times (26). We identified temporary periods of directed motion within otherwise diffusive trajectories (64% of all directed trajectories; n = 59). Their duration had a single-exponential distribution with a mean time τ = 4.0 s (26), indicative of a one-step process. The MSD computed for the periods identified as purely diffusive was linear when plotted against time (SI Fig. 13), establishing the consistency of our analysis.
Taken together, these data provide a simple picture of receptor motion. During periods of free Brownian diffusion, the GABAARs “explore” an extended part of the membrane, whereas, upon binding to MTs, they are displaced in a directed manner until they dissociate from the MTs and resume their Brownian motion. Such dynamics is referred to as a conveyor belt mode of motion (25).
Asymmetric Transport of the Receptors.
We studied the dynamics of individual GABA receptors when neurons were exposed to an external GABA signal. In a uniform concentration of GABA (10 μM) the characteristics of receptor movement in the C-region of GCs were unchanged, with the mean diffusion coefficient Dm, velocity vm, and association time τ equal to 0.25 ± 0.02 μm2·s−1, 0.28 ± 0.02 μm·s−1, and 3.9 s, respectively, close to their values in the absence of GABA. In the presence of a GABA gradient both the diffusion (Dm = 0.23 ± 0.03 μm2·s−1; n = 22) and the velocity (vm = 0.24 ± 0.03 μm·s−1) of the receptor movement were not modified either. However, the asymmetric stimulation induced a significant change in the orientation of the trajectories (measured by the angle between the GC axis and the axis defined by the first and last points of the trajectory). At t = 0 the angular distribution was symmetric with respect to the GC axis (Fig. 3F). After a 10-min exposure, the distribution became asymmetric with the mean orientation pointing toward the GC edge facing the gradient (Fig. 3G). These results support the notion that GABAARs are actively displaced toward the GABA source by means of oriented conveyor belt motions, leading to a polarized distribution in the membrane.
Enhancement of the Asymmetry of Intracellular Calcium.
We next investigated whether the relocalization of the membrane chemoreceptors (GABAARs) had implications on downstream intracellular signaling. We measured the concentration of intracellular calcium, an important factor in axon pathfinding (23) and in GABA signaling. At early stages of development of the nervous system, activation of GABAARs leads to membrane depolarization (28), which may trigger calcium entry through voltage-dependent calcium channels (23, 24). Variations of intracellular calcium concentration [Ca2+] were detected by measuring the fluorescence ratio F (Fig. 4 A and B) of the dyes Fluo-4 and Calcein Orange (a calcium-insensitive dye used to normalize the signal), which were loaded 30 min before the experiment and imaged simultaneously with a DualView setup. In GCs subjected to a gradient, F integrated over the entire GC increased and saturated after ≈5–10 min (Fig. 4C). The gradient of [Ca2+] within the cell was analyzed by computing the ratio R = Fprox/Fdist of the signal integrated over the proximal and distal regions (Fig. 4D). R(t) first rose slowly and saturated around R = 1 + 0.05. Then, after Te = 11 ± 2 min, R increased dramatically and reached a value close to 1 + 0.17 at 18 min (Fig. 4D). The fact that Te is close to Tr supports the notion that the increase in [Ca2+] asymmetry is related to the receptor redistribution. This was confirmed with calcium measurements after nocodazole treatment preventing the receptor redistribution. In this case there was no asymmetry in GABAAR localization, and the rapid increase of R was abolished (Fig. 4D). The slow component in the time evolution of the ratio R(t) can thus be attributed to the intrinsic asymmetry imposed by the external gradient, and the fast rise can be attributed to an additional effect induced by the spatial rearrangement of GABAARs. Our data therefore provide evidence that the asymmetric relocalization of GABAAR leads to amplification in the asymmetry of downstream signaling.
Fig. 4.
Intracellular calcium measurements. Image of the fluorescence ratio, F, of Fluo-4 to calcein orange at t = 0 (A) and after 20 min (B). (Scale bar: 10 μm.) The proximal and distal regions are indicated by dashed and dotted lines, respectively. (C) Relative change of ΔF/F = [F(t) − F(0)]/F(0) in the total calcium signal in the GC (averaged over 10 GCs). (D) Time evolution of R = Fprox/Fdist (averaged over 10 GCs) in cells exposed to a GABA gradient in the absence (blue triangles) and presence (red squares) of nocodazole. The gray line, used as a guide for the eye, is an adjustment by the curve R(t) − 1 = A1t/(t + B1) + A2tn/(tn + B2n). The ratio of the calcein orange signals in the proximal and distal regions (black squares) remains close to 1, showing that the variations of R are only due to changes in the [Ca2+] gradient.
Discussion
MT-Dependent Redistribution of GABAARs.
Immunofluorescence images on fixed cells show that GABAARs are asymmetrically localized in GCs subjected to a GABA gradient, with an increased concentration in the proximal region. To address the dynamic processes that induce this polarized distribution, we used a single-molecule imaging approach and analyzed the membrane dynamics of QD-tagged GABAARs. We observe that GABAARs redistribute across the GC toward the gradient source in the presence of a chemoattractant GABA gradient. Concomitant with this relocalization in the membrane, the intracellular calcium response displays an enhanced asymmetry between the proximal and distal regions, indicative of amplification in gradient sensing. Because the redistribution takes place on a time scale of ≈10 min, shorter than the time required for GC steering, it points to asymmetric relocalization as an initial amplification step in the detection of GABA gradients.
The asymmetric relocalization of signaling molecules during chemotaxis has been mostly investigated in nonneuronal systems, such as neutrophils and the amoeba D. discoideum. These studies indicate a situation different from what we observed for GABAARs. In Dictyostelyum amoebas, for instance, the presence of a chemoattractant cAMP gradient leads to a cytoskeleton-independent polarized reorganization of cytoplasmic molecules downstream of the signaling pathway (11), such as pleckstrin homology domain-containing proteins [such as cytosolic regulator of adenylyl cyclase (29)] due to the asymmetric translocation to the membrane of phosphatidylinositol 3-kinase and PI-phosphatase PTEN. cAMP receptors remain, on the contrary, homogeneously distributed in the membrane although, as revealed by single-molecule measurements, the kinetics of their association/dissociation to the chemoattractant (30) can be modulated according to their location in the cell.
Compared with chemotactic systems such as amoebas or leukocytes, neuronal cells respond to a larger variety of chemical cues (1–3). It is then essential that the GC can specifically amplify an external signal by redistributing the corresponding receptors. The absence of redistribution of GABAARs for GCs submitted to an Ach gradient or in a gabazine bath indicates that GABAAR relocalization requires their activation. However, an asymmetric activation is not sufficient, as indicated by the absence of redistribution when MTs are depolymerized (Fig. 2C). Moreover, an asymmetric activation of the receptors is not necessary to achieve their polarized distribution, provided that an asymmetric stimulation of downstream elements of the signaling pathway (for instance, the cytoskeleton) is performed, as shown by the redistribution of GABAARs submitted to both an Ach gradient and a GABA bath (Fig. 2A). This observation also illustrates the possible interplay between different signaling pathways (GABA and Ach), which could be a way for GCs to finely tune their in vivo response.
Mechanism of Active Transport.
A few studies have previously noted the ability of the GC to spatially rearrange its membrane components in response to a gradient of guidance cues. Guirland et al. (13) have reported an asymmetry in the localization of lipid rafts and raft-associated tyrosine kinase B receptors after exposure to a brain-derived neurotrophic factor (but not glutamate) gradient. In these experiments the asymmetric distribution, however, was determined by immunostaining on fixed cells, preventing an analysis of the dynamics of the redistribution. The mechanism leading to the asymmetry is not fully established, and, in particular, it is not known whether the asymmetric distribution resulted from lateral motion in the membrane or from insertion by exocytosis of additional molecules.
Single QD measurements have provided detailed insight into the mechanisms responsible for asymmetric redistribution of GABAARs. Within the GC membrane GABAARs display a rapid thermal motion, with a coefficient D ≈ 0.25 μm2/s. Because Brownian dynamics alone leads to a uniform distribution in the membrane, a polarized localization of receptors requires a mechanism able to counteract the effect of diffusion. In principle, the asymmetric redistribution could be achieved by a “diffusion trap” mechanism in which receptors diffuse and are transiently captured in sites asymmetrically distributed in the GC, as shown for the postsynaptic accumulation of neurotransmitter receptors (31). However, this mechanism is not supported by our data because no trapping of GABAARs was observed on individual trajectories during application of the gradient. Our results indicate that asymmetric redistribution can be achieved if GABAARs are transiently “pushed” toward the leading edge. Combined with diffusion, these oriented motions result in a receptor distribution biased toward the gradient source.
Single-particle tracking is a simple way to characterize molecular movements by computing the MSD vs. time on individual trajectories. In the actin-rich P-region of the GCs GABAARs diffused freely and the actin meshwork did not directly influence the receptor movements. In contrast, the implication of the MTs in the regulation of the receptor lateral dynamics was attested by two observations: (i) the redistribution was abolished after nocodazole treatment, and (ii) MT-dependent directed movements of the GC were revealed by the analysis of receptor trajectories specifically in the MT-rich C-region. The direct or indirect physical interactions of GABAARs with MTs provide a coupling of the sensing molecules to cytoskeleton elements that have an important role in GC architecture, steering, and motility (10, 32). Our taxol experiments indicate that MT-dependent movements are not mediated by molecular motors but rather suggest that the binding site with GABAARs is at the extremity of MTs. The regulation of MT dynamics during guidance is actually thought to occur through modifications of their extremities by anisotropic activity of the + end tracking proteins (+TIPS), which promote asymmetric MT stabilization and growth (32–35). Moreover, because activation of the receptors is required for their relocalization, coupling between GABAARs and MTs is probably regulated during guidance through a molecular mechanism that remains to be elucidated.
Model of Autocatalytic Amplification in Gradient Sensing.
Altogether, our results support the following model for the GABAAR redistribution induced by their coupling to MT dynamics and for the possible role of this polarization in the amplification of gradient sensing (Fig. 5). At t = 0 the receptors are symmetrically distributed and move without a preferred orientation. A gradient of chemoattractant then induces an activation gradient of GABAARs and, consequently, a local asymmetry in intracellular [Ca2+] in the GCs. This asymmetry results in a remodeling of the cytoskeleton and preferential growth of the MTs toward the leading edge of the GC (10, 32, 36). As a result of the MT-dependent conveyor belt motion, the GABAAR movements map the MT elongation dynamics and the receptors are displaced toward the side of the GC facing the gradient. Concentrating the receptors in the proximal region further reinforces the asymmetric calcium response. Consequently, calcium-dependent interactions between receptors and MTs together with the initial asymmetric activation of GABAARs create a positive feedback loop that polarizes the distribution of GABAARs in the GC membrane and amplifies the asymmetry in the calcium response. When the GABA gradient is released there is no more asymmetric stimulation and the receptors diffuse back to their initial symmetric distribution.
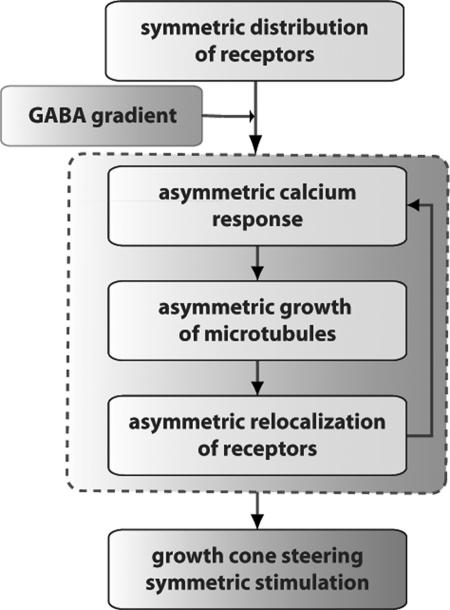
Fig. 5.
Model of amplification by positive feedback. A gradient of GABA triggers an asymmetric calcium response leading to asymmetric elongation of the MTs. This induces redistribution of GABAARs, which further enhances the asymmetry in the calcium and cytoskeleton response. This positive feedback loop (dashed gray line) leads to amplification of gradient sensing and ultimately to GC steering and elongation toward the source of chemoattractant.
An important finding in our study is the observation of a conveyor belt motion in which GABAARs alternate between MT-dependent movement and free diffusion. Brownian motion, which neither requires specific molecular interactions nor consumes energy, is an essential element to account for the ability of receptors to rapidly explore their environment. In the GC response, diffusion plays a crucial role in the amplification and filtering of the external signals as well as in the adaptation of the cell to environmental changes. Amplification results from the mobility of receptors and their interaction with molecular partners, leading to an autocatalytic relocalization mechanism. Temporal filtering is achieved because the lateral motion of the receptors contributes to the kinetics of their redistribution, which is, at least partly, diffusion-limited. Because the redistribution takes ≈10 min, it acts as a low-pass filter preventing faster fluctuations from being interpreted as guidance signal and contributes to the robustness in the detection and amplification process. Finally, receptor diffusion is essential for the cellular adaptation to environmental changes because it provides a simple mechanism for the rearrangement of the sensing machinery in the membrane if, for instance, the gradient orientation is modified.
Conclusion
Our results show that concentration gradients of extracellular cues are not only detected by the sensing apparatus of the GC membrane but also can trigger its spatial reorganization, which leads to a change in the asymmetry of intracellular calcium concentration. This sequence of events (detection, reinforcement, and propagation of the spatial cue) is characteristic of the formation of cell polarity in many cellular systems (37). By increasing the relative concentration of receptors in a particular region of the GC and, consequently, depleting the remaining membrane, the asymmetric redistribution of GABAARs has an effect similar to the local self-enhancement and global inhibition mechanism proposed for chemotactic amplification in eukaryotic cells (12). Redistribution of receptors occurs through lateral dynamics in the membrane, a mechanism very different from the one proposed for the polarization of signaling molecules in Dictyostelyum amoebas or neutrophils (11). As a result, the redistribution of receptors in GCs takes place over ≈10 min, much longer than the ≈1 s needed for the relocalization of intracellular molecules in Dictyostelyum amoebas. This could reflect the difference in the physiological role of gradient sensing in these systems: in GCs the addressing needs to be accurate but not necessarily rapid, whereas in amoebas hunting and food-searching require an almost instantaneous response. Given its simplicity and sensitivity, our single-molecule assay should be useful to investigate the establishment of cell polarity and formation of membrane heterogeneities in many neuronal or nonneuronal systems.
Materials and Methods
Labeling, Imaging, and Tracking with QDs.
The γ2 subunit of the GABAAR was sequentially labeled on the membrane of spinal neurons of rat (embryonic day 14, 3–6 days in vitro). Neurons were incubated in cell culture medium (MEM without phenol red supplemented with 4 mM NaHCO3, 10 mM Hepes buffer, 6 g/liter glucose, 2 mM glutamine, 1 mM Na+ pyruvate, and 1× B27 supplement) at 37°C first with a specific anti-γ2 antibody raised in guinea pig (a gift from J. M. Fritschy, Univ. Zurich, Switzerland) for 10 min then with a biotinylated anti-guinea pig antibody (1 μg/ml; Rockland Immunochemicals, Gilbertsville, PA) for 10 min. GCs were then labeled with streptavidin-coated QDs (QD605Sav, 1-min incubation, 0.25 nM; Quantum Dot, Carlsbad, CA), and rinsed with culture medium. QDs were imaged by epifluorescence microscopy by using an Hg lamp (excitation filter 525AF45, dichroic filter 560DRLP, emission filter 605NB20; Omega Opticals, Brattleboro, VT), and an oil immersion objective (×60, N.A. = 1.40; Olympus). The fluorescence was collected on a CCD camera (CoolSnap ES; Roper Scientific, Tucson, AZ).
Calcium Imaging.
Neurons were loaded with 1 μM Fluo4 and 4 μM calcein orange (both from Molecular Probes, Carlsbad, CA) in MEM for 20 min at 37°C and then washed extensively in MEM. Calcein and Fluo4 were simultaneously excited (480DF30 filter; Omega Opticals). Their emission was detected with a DualView setup (Optical Insights, Tucson, AZ) with appropriate filters (dichroic filter 565 nm, emission filters at 530 and 630 nm) and projected onto the two halves of the CCD chip. Image sequences were acquired in a time-lapse mode at 0.125 Hz (acquisition time: 100 ms).
Supplementary Material
Acknowledgments
We thank our colleagues Y. Bellaiche, J.-L. Bessereau, S. Bonneau, A. Chedotal, P. Desbiolles, S. Dieudonné, S. Lévi, and A. Prochiantz for useful discussions and critical reading of the manuscript. This work was supported by the Action Concertée Interface Physique-Chimie–Biologie: Dynamique et Réactivité des Assemblages Biologiques, the Fondation pour la Recherche Médicale, and the French–Israeli Program.
Abbreviations
- GC
growth cone
- Ach
acetylcholine
- QD
quantum dot
- MT
microtubule
- MSD
mean-square displacement
- GABAAR
GABAA receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702536104/DC1.
References
- 1.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 2.Song H, Poo M. Nat Cell Biol. 2001;3:E81–E88. doi: 10.1038/35060164. [DOI] [PubMed] [Google Scholar]
- 3.Guan KL, Rao Y. Nat Rev Neurosci. 2003;4:941–956. doi: 10.1038/nrn1254. [DOI] [PubMed] [Google Scholar]
- 4.Gundersen RW, Barrett JN. Science. 1979;206:1079–1080. doi: 10.1126/science.493992. [DOI] [PubMed] [Google Scholar]
- 5.Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 6.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 7.Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C. Nature. 2005;438:94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng JQ, Felder M, Connor JA, Poo MM. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 9.Rosoff WJ, Urbach JS, Esrick MA, McAllister RG, Richards LJ, Goodhill GJ. Nat Neurosci. 2004;7:678–682. doi: 10.1038/nn1259. [DOI] [PubMed] [Google Scholar]
- 10.Dent EW, Gertler FB. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 11.Van Haastert PJ, Devreotes PN. Nat Rev Mol Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- 12.Meinhardt H. J Cell Sci. 1999;112:2867–2874. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- 13.Guirland C, Suzuki S, Kojima M, Lu B, Zheng JQ. Neuron. 2004;42:51–62. doi: 10.1016/s0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- 14.Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ. Nat Neurosci. 2006;9:1265–1273. doi: 10.1038/nn1773. [DOI] [PubMed] [Google Scholar]
- 15.Leung KM, van Horck FP, Lin AC, Allison R, Standart N, Holt CE. Nat Neurosci. 2006;9:1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang Y, Li Y, Zhang Z, Cui K, Wang S, Yuan XB, Wu CP, Poo MM, Duan S. Nat Neurosci. 2002;5:843–848. doi: 10.1038/nn899. [DOI] [PubMed] [Google Scholar]
- 17.Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 18.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Mohler H. Proc Natl Acad Sci USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck KB, Zheng JQ. J Neurosci. 2002;22:9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coyle JE, Qamar S, Rajashankar KR, Nikolov DB. Neuron. 2002;33:63–74. doi: 10.1016/s0896-6273(01)00558-x. [DOI] [PubMed] [Google Scholar]
- 22.Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez TM, Zheng JQ. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- 24.Fukura H, Komiya Y, Igarashi M. J Neurochem. 1996;67:1426–1434. doi: 10.1046/j.1471-4159.1996.67041426.x. [DOI] [PubMed] [Google Scholar]
- 25.Saxton MJ, Jacobson K. Annu Rev Biophys Biomol Struct. 1997;26:373–399. doi: 10.1146/annurev.biophys.26.1.373. [DOI] [PubMed] [Google Scholar]
- 26.Bouzigues C, Dahan M. Biophys J. 2007;92:654–660. doi: 10.1529/biophysj.106.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. J Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein V, Nicoll RA. Neuron. 2003;37:375–378. doi: 10.1016/s0896-6273(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 29.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 30.Ueda M, Sako Y, Tanaka T, Devreotes P, Yanagida T. Science. 2001;294:864–867. doi: 10.1126/science.1063951. [DOI] [PubMed] [Google Scholar]
- 31.Choquet D, Triller A. Nat Rev Neurosci. 2003;4:251–265. doi: 10.1038/nrn1077. [DOI] [PubMed] [Google Scholar]
- 32.Kalil K, Dent EW. Curr Opin Neurobiol. 2005;15:521–526. doi: 10.1016/j.conb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Engel U, Rusch J, Scherrer S, Sheard K, Van Vactor D. Neuron. 2004;42:913–926. doi: 10.1016/j.neuron.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Wittmann T, Waterman-Storer CM. J Cell Biol. 2005;169:929–939. doi: 10.1083/jcb.200412114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou FQ, Waterman-Storer CM, Cohan CS. J Cell Biol. 2002;157:839–849. doi: 10.1083/jcb.200112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drubin D. Cell Polarity. Oxford: Oxford Univ Press; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.