Abstract
Testis-derived testosterone has been recognized as the key factor for morphogenesis of the Wolffian duct, the precursor of several male reproductive tract structures. Evidence supports that testosterone is required for the maintenance of the Wolffian duct via its action on the mesenchyme. However, it remains uncertain how testosterone alone is able to facilitate formation of regionally specific structures such as the epididymis, vas deferens, and seminal vesicle from a straight Wolffian duct. In this study, we identified inhibin beta A (or Inhba) as a regional paracrine factor in mouse mesonephroi that controls coiling of the epithelium in the anterior Wolffian duct, the future epididymis. Inhba was expressed specifically in the mesenchyme of the anterior Wolffian duct at embryonic day 12.5 before the production of androgens. In the absence of Inhba, the epididymis failed to develop the characteristic coiling in the epithelium, which showed a dramatic decrease in proliferation. This loss of epididymal coiling did not result from testosterone deficiency, because testosterone production and parameters for testosterone action such as testis descent and anogenital distance remained normal. We further found that initial Inhba expression did not require testosterone as Inhba was also expressed in the anterior Wolffian duct of female embryos where no testosterone was produced. However, Inhba expression at later stages depended on testosterone. These results demonstrated that Inhba, a mesenchyme-specific gene, acts collectively with testosterone to facilitate epididymal coiling by stimulating epithelial proliferation.
Keywords: activin, androgen, epididymis, Wolffian duct
Alfred Jost (1, 2) first demonstrated in the 1940s and 1950s that testosterone is required for the maintenance of the Wolffian duct, the precursor of several male reproductive tract structures. Castration of male rabbit embryos resulted in a complete regression of the Wolffian duct, and testosterone replacement was able to restore the development of the Wolffian duct. The critical role of androgen signaling in establishment of the male reproductive tract was further confirmed by null mutations of the androgen receptor (AR) as seen in human cases of androgen insensitivity syndrome and in the Tfm (testis feminization) mouse. In these examples, the XY individuals developed as pseudohermaphrodites with degenerated Wolffian ducts and internal testes (3–5). Furthermore, administration of antiandrogens to pregnant females suppressed development of internal and external genitalia of male offspring (6, 7). This evidence cements the paradigm of sexual differentiation that claims testosterone as the only testis-derived factor essential for the establishment of the male reproductive tract.
The mechanism of testosterone action on the Wolffian duct involves the interaction between epithelium and mesenchyme. In mouse embryos, AR first appeared in the mesenchyme surrounding the Wolffian duct. Conversely, AR was not expressed in the ductal epithelium until embryonic day 15.5 (E15.5), when the ductal morphogenesis starts (8). In the absence of androgens, Wolffian duct mesenchyme underwent degeneration via apoptosis, indicating that androgens stabilize Wolffian duct development by directly acting on the Wolffian duct mesenchyme (9). The actions of androgen on the Wolffian duct epithelium, on the other hand, appear to be indirect via a mesenchyme-derived regulator(s). When the upper Wolffian duct epithelium (the future epididymis) was grafted onto the lower Wolffian duct mesenchyme (the future seminal vesicle), the epithelium underwent seminal vesicle morphogenesis and expressed markers specific for seminal vesicle epithelium instead of those for epididymal epithelium (10). This inductive ability of Wolffian duct mesenchyme was also found in the prostate, providing further support that AR in the mesenchyme is necessary to dictate androgenic actions of the epithelium (11). Furthermore, when the epithelium from the AR-deficient testicular feminization mutation mouse was recombined with wild-type prostatic mesenchyme the AR-deficient epithelium still underwent ductal morphogenesis, proliferation, and cytodifferentiation resembling the prostate (12, 13). These experiments together demonstrate that morphogenesis of the Wolffian duct requires direct androgen action on the mesenchyme to maintain its survival and a mesenchyme–epithelium interaction to determine the regional specialization of the epithelium.
The goals of the current research were to identify a putative mesenchyme-derived factor that has a functional role in regulating Wolffian duct morphogenesis. Our preliminary data demonstrated that inhibin beta A (Inhba) was expressed specifically in the mesenchyme of the anterior Wolffian duct, the future epididymis (14). Inhba, a subunit of both inhibins and activins, has been shown to be involved in ductal morphogenesis of the kidney and prostate during embryonic development (15, 16). We therefore hypothesized that Inhba was a potential candidate for the mesenchyme-derived gene responsible for transforming a straight Wolffian duct into the convoluted and coiled structure of the epididymis.
Results
Expression of Inhba and Phospho-SMAD2/3 During Wolffian Duct Development.
To identify genes that may be involved in Wolffian duct differentiation, we performed in situ hybridization screening on various signaling molecules such as members of the TGF-β family. We found that mRNA for Inhba was expressed in the anterior and middle portions of the mesonephros or the future epididymis (Fig. 1A). Expression of Inhba was also found in the testis as described (14). At E15.5, when Wolffian duct morphogenesis commenced, Inhba mRNA was exclusively expressed in the mesenchyme but not the epithelium of the Wolffian duct (Fig. 1A). This mesenchyme-specific pattern of Inhba expression persisted in mesonephroi at E17.5 and E19.5, as the anterior Wolffian duct began to coil and take on the appearance of the adult epididymis (data not shown). Because Inhba is a critical subunit of activins and inhibins (antagonists of activins), we investigated whether the activin pathway was active in the Wolffian duct. We examined the intracellular effectors of activins, phospho-SMAD2/3, by immunocytochemistry. Phospho-SMAD2/3 was detected in nuclei of the Wolffian duct epithelium, but not the mesenchyme, at E15.5, E17.5, and E19.5 (representative image seen in Fig. 1B). These observations together suggest that the Wolffian duct mesenchyme is a source of activins, which could act on the Wolffian duct epithelium.
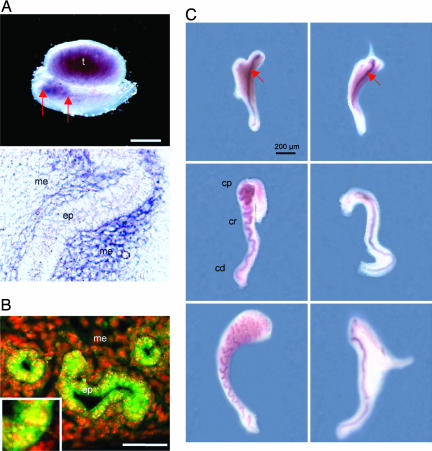
Fig. 1.
Functional roles of Inhba in mouse epididymal coiling. (A) Expression of Inhba mRNA in whole-mount (Upper) and sectioned (Lower) mesonephroi at E15.5. Positive staining (red arrows) appears as dark purple. Anterior of the embryo is on the left. (B) Immunocytochemistry for phospho-SMAD2/3 (green) counterstained with nuclear marker DAPI (red) in the Wolffian duct at E15.5. (Inset) A higher magnification (×40) of a portion of the epithelium is shown to demonstrate the speckled nuclear staining of the phospho-SMAD2/3. (C) Whole-mount in situ hybridization for Pax2, an epithelial cell marker for the Wolffian duct, on mesonephroi without testes attached on wild-type (Left) and Inhba−/− (Right) embryos at E15.5 (Top), E17.5 (Middle), and E19.5 (Bottom). Specific staining appears as dark purple (red arrows and magnification was ×4). cp, caput; cr, corpus; cd, cauda; ep, epithelium; me, mesenchyme; t, testis. (Scale bars: 500 μm, A; 100 μm, B.)
Defects in Epididymal Coiling in Inhba−/− Embryos.
To investigate whether Inhba has a functional role in Wolffian duct differentiation, we examined the development of the Wolffian duct in wild-type and Inhba−/− embryos by using whole-mount in situ hybridization for Pax2, a marker for the Wolffian duct epithelium (17). At E15.5, the Wolffian duct remained as a straight tube in the mesonephroi of both wild-type and Inhba−/− male littermates (Fig. 1C). At E17.5 and E19.5, significant coiling became apparent in the developing epididymides of wild-type embryos (Fig. 1C); however, this coiling was completely absent in all three regions (caput, corpus, and caudal) of the developing epididymides in the Inhba−/− male embryos (Fig. 1C). In the Inhba−/− embryos, the Wolffian duct failed to elongate and coil, leaving a straight duct in the mesonephros that resembled that of the E15.5 embryos. The Inhba heterozygous animals were fertile, and their epididymides were indistinguishable from those of wild-type animals (data not shown).
Parameters of Androgen Action in the Inhba−/− Male Embryos.
Although the Wolffian duct failed to coil in the absence of Inhba, the Wolffian duct was still present in the mesonephros, suggesting that androgen action with regard to maintenance of the Wolffian duct was normal. To test this, we examined parameters for androgen action, including Leydig cell differentiation, testis descent, anogenital distance, and testosterone production. First, we found that the expression of P450 side-chain cleavage enzyme (P450 Scc), a steroidogenic marker for Leydig cells, was present in both the wild-type and Inhba−/− testis (Fig. 2A). Second, we isolated the entire urogenital tract at E19.5 to examine testis descent, a process controlled by androgens. The Inhba−/− testes descended to the lateral side of the bladder similarly to their wild-type littermates (Fig. 2A). The anogenital distance, which is normally greater in males than females because of androgen action, was similar in wild-type, Inhba+/−, and Inhba−/− male embryos (Fig. 2A). Last, we measured the testosterone content in individual testis from wild-type, Inhba+/−, and Inhba−/− newborns and found no statistical differences among phenotypes [supporting information (SI) Fig. 5; n = 8]. We also examined the development of prostate and seminal vesicles at birth and found no differences between the wild-type and Inhba−/− animals (data not shown).
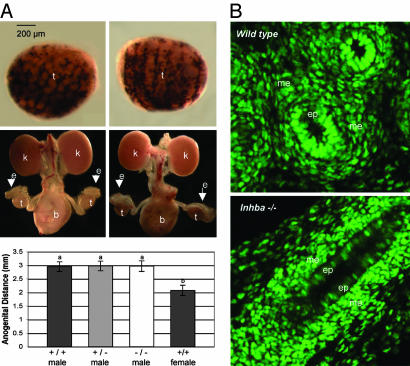
Fig. 2.
Parameters of androgen action in wild-type and Inhba−/− embryos. (A) P450 Scc expression in the testis, testis descent, and anogenital distance were examined. (Top) Whole-mount in situ hybridization for P450 Scc, a marker for Leydig cells, was performed on wild-type (Left) and Inhba −/− (Right) littermate testis (black staining). (Middle) Light microscopic images of the entire urogenital system were taken from wild-type (Left) and Inhba−/− (Right) embryos to monitor testis descent. (Bottom) Anogenital distance of newborns was measured, and each bar represents the mean ± SEM of male Inhba+/+ (n = 8), +/− (n = 16), and −/− (n = 8) males compared with wild-type females (n = 30). Statistical significance between each symbol (a and b) was P < 0.01. (B) The presence of AR was detected by immunocytochemistry (green) in the wild-type (Upper) and Inhba−/− (Lower) epididymides at E19.5. b, bladder; e, epididymis; ep, epithelium; k, kidney; me, mesenchyme; t, testis.
We further investigated whether the responsiveness of the Wolffian duct to androgens (AR expression) remained intact in the absence of Inhba. By using immunocytochemistry for AR, we found that AR proteins were restricted to the mesenchyme, and minimal staining was found in the epithelium at E15.5 in wild-type mesonephroi as described (8). At E17.5 and E19.5 AR continued to be expressed in the mesenchyme and also began to appear in the epithelium (representative image shown in Fig. 2B). Inhba−/− embryos retained the mesenchyme expression of AR at E15.5 and other stages similar to the wild type. However, at E17.5 and E19.5, when AR expression became strongly positive in the Wolffian epithelium in the wild type, only a low level of AR was detected in a few epithelial cells in the Inhba−/− Wolffian duct (representative image shown in Fig. 2B). Taken together, these results suggest that loss of Inhba did not alter testosterone production or AR expression in the Wolffian duct mesenchyme, the primary target of androgens. However, normal epithelial expression of AR after E15.5 appeared to require the presence of Inhba in the Wolffian duct mesenchyme.
Mechanisms of Inhba Action in Epididymal Coiling.
Histological evaluation of E19.5 wild-type and Inhba−/− epididymides provided more insight into the regionally specific defects in Wolffian duct differentiation. The reduction in ductal coiling was evident by the decreased number of ductal cross-sections within the epididymides of Inhba−/− embryos compared with wild type (Fig. 3A). In the wild-type epididymis, the high cuboidal to low columnar epithelial layer was surrounded by a well organized mesenchyme (Fig. 3A). In Inhba−/− embryos, the epithelial layer became high columnar in shape, and cell width appeared to be decreased as the epithelial layer became crowded. The mesenchyme also exhibited abnormalities as the mesenchymal cells surrounding the duct were condensed and disorganized (Fig. 3A).
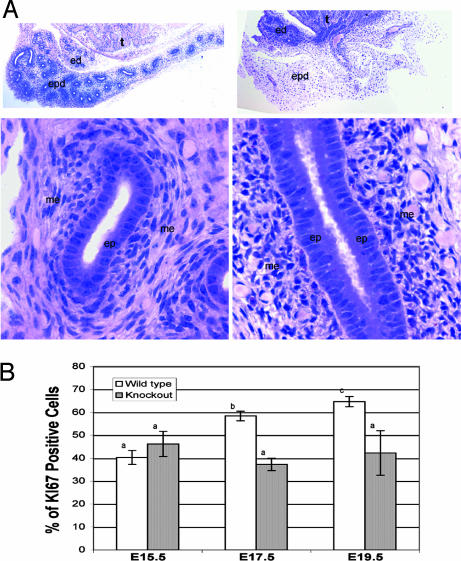
Fig. 3.
Cellular and proliferation defects in the Inhba−/− epididymides. (A) Periodic acid/Schiff staining of the E19.5 epididymides in wild-type (Left) and Inhba−/− (Right) littermates. (Upper) Magnification: ×4. (Lower) Magnified sections (×20) of corpus portion of the epididymis are shown. ed, efferent ducts; ep, epithelium; epd, epididymis; me, mesenchyme; t, testis. (B) Proliferation in the epididymal epithelia of wild-type (white bars) and Inhba−/− (gray bars) embryos at E15.5, E17.5, and E19.5. Proliferation was measured by immunostaining for Ki67. For each tissue, serial sections were obtained and stained, and at least 10 sections (24 μm apart) were analyzed. The percentage proliferation was calculated as the fraction of Ki67-positive cells over the total number of cells based on total nuclear staining (DAPI staining). The sample size was three embryos for each genotype and statistical significance between each symbol (a, b, and c) was P < 0.01.
The dramatic transformation of a straight Wolffian duct into a convoluted epididymal structure from E15.5 to E19.5 requires expansion of the epithelium. We speculated that either a decrease in epithelial proliferation, an increase of epithelial apoptosis, or a combination of both could contribute to the loss of epididymal coiling in Inhba−/− embryos. We measured proliferation and apoptosis by immunostaining for Ki67 and TUNEL assay, respectively. In the wild-type embryos, a significant increase in the percentage of total Ki67-positive cells in the entire Wolffian duct epithelium was observed during development from E15.5 (40%), E17.5 (57%), to E19.5 (65%; Fig. 3B). In the Inhba−/− embryos, this increasing trend was absent as the percentage of total Ki67-positive cells remained similar from E15.5 to E19.5. At E15.5, the percentage of total Ki67-positive cells in the entire Wolffian duct epithelium was not different between wild-type and Inhba−/− embryos (Fig. 3B). However, at E17.5 and E19.5, when wild-type epithelium showed an increase in proliferation, the percentage of Ki67-positive cells in the Inhba−/− epithelium remained similar to that of E15.5 (Fig. 3B). Apoptosis was also examined in the wild-type and Inhba−/− epididymides from E15.5 to E19.5. We found that apoptotic cells were rare in all genotypes, and no difference was observed (data not shown). These results suggest that Inhba is responsible for proper epithelial proliferation in the Wolffian duct, which is essential for epididymal coiling.
In addition to the epididymal phenotypes, defects were found in the testis of the Inhba−/− embryos. This observation raised the possibility that the epididymal phenotypes could be secondary to the testis defects, instead of the loss of local production of Inhba. To test this possibility, we examined markers for Sertoli cells (AMH or anti-Müllerian hormone and SOX9 or Sry-related HMG box 9) and Leydig cells (Cyp17) and found no differences between wild-type and Inhba−/− testes (SI Fig. 6; n = 6). Along with the finding on normal testosterone production in Inhba testes and existing evidence in the field (see Discussion), we concluded that the epididymal phenotypes were not caused by defects in the testes.
Regulation of Inhba Expression in the Wolffian Duct.
The overlapping patterns of Inhba (Fig. 1A) and AR (Fig. 2B) expression in the Wolffian duct mesenchyme suggested that Inhba expression could be regulated by androgens. To test this possibility, we first examined expression of Inhba in both male and female mesonephroi at E12.5 when androgen-producing fetal Leydig cells have not yet appeared in the testis. At E12.5, Inhba expression was present in both male and female mesonephroi in an identical pattern: strong expression in the anterior Wolffian duct (Fig. 4A). These results indicated that initial expression of Inhba in the Wolffian duct mesenchyme is not male-specific and does not require testosterone.
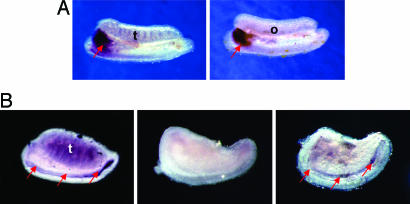
Fig. 4.
Regulation of Inhba expression in the Wolffian duct. (A) Inhba expression in E12.5 male (Left) and female (Right) mesonephroi and gonads by whole-mount in situ hybridization. (B) Inhba expression in the mesonephros with testis attached with vehicle treatment (methanol) (Left), mesonephros alone with vehicle (methanol) (Center), and mesonephros alone with testosterone (10−7 M in methanol) (Right) for 48 h starting at E13.5. Positive staining (red arrows) appears as dark purple or brownish color. The sample size for all experiments was three repetitions for each treatment. o, ovary; t, testis. (Magnification: ×4.)
To investigate whether Inhba expression at later stages of development requires testosterone or the presence of testis, we cultured E13.5 mesonephroi with testes attached, mesonephroi alone with methanol (vehicle for testosterone), or mesonephroi alone with testosterone (10−7 M in methanol; Fig. 4B). E13.5 was chosen, as it was the stage when testosterone production began to peak. After 48 h of culture, we found that when the testis remained attached to the mesonephros, Inhba expression in the anterior Wolffian duct extended posteriorly, similar to what was seen at the same stage in vivo. However, in mesonephroi cultured alone without the testosterone supplement, Inhba expression in the Wolffian duct was significantly reduced (Fig. 4B). Addition of testosterone only partially restored the Inhba expression in the absence of the testis. These data suggest that testosterone and/or other factors from the testis are essential for the maintenance of Inhba expression after E13.5.
Discussion
Formation of the tubal structure in organs such as the lung, kidney, and mammary gland is established through a universal mechanism of mesenchyme–epithelium interaction (18–20). Here, we demonstrate that morphogenesis of the male reproductive tract, specifically the epididymis, also requires a cross-talk between mesenchyme and epithelium. It has long been proposed that a mesenchyme-derived factor dictates the differentiation of the epithelium in the Wolffian duct (21). Although expression profiling and in vitro experiments have suggested the potential involvement of several candidate molecules such as prostaglandin E2 (22), epidermal growth factor (23), keratinocyte growth factor (24), and neurotrophins (25) in this process, the in vivo role of these putative inductive factors has never been confirmed. Alarid et al. (26), using transplantation technique, demonstrated that basic fibroblast growth factor (bFGF) antibody caused degeneration of the genital ridge and loss of epididymis, suggesting that bFGF is important for maintaining the Wolffian structure (26). However, a genetic model remains to be developed to confirm the role of bFGF in vivo. In this research, we discovered Inhba as a mesenchyme-specific gene that controls regional differentiation of the epididymis. The mesenchyme-derived Inhba is responsible for proliferation of the epididymal epithelium, leading to the transformation of the straight Wolffian duct into the convoluted and coiled structure of the epididymis.
Inhba Is a Mesenchyme-Derived Gene that Is Required for Epithelial Coiling of the Epididymis.
Inhibin beta genes (Inhba and Inhbb) encode inhibin beta proteins (INHBA and INHBB), which are subunits of inhibins (dimers of inhibin alpha and beta subunits) and activins (dimers of inhibin beta subunits) (27, 28). The presence of Inhba mRNA, but not Inhbb mRNA (shown in ref. 14), in the anterior Wolffian duct mesenchyme suggests that inhibin A (heterodimers of INHBA and the alpha subunit) and/or activin A (homodimers of INHBA) are the possible products in this region. Activins, but not inhibins, are known to induce cellular responses via activin receptors and the SMAD2/3 pathway (29–31). Inhibins function to antagonize activins through either competition of receptor binding (32) or betaglycan (33). The expression of activin receptor IIB (34) and the presence of phospho-SMAD2/3 (this paper) in the Wolffian epithelium suggest that the activin pathway is activated and targeted to the Wolffian epithelium. Defects in epithelial proliferation in the Inhba−/− anterior Wolffian duct provide further functional evidence to support that mesenchyme-derived activin A stimulates epithelium expansion in the epididymis. The presence of INHBA mRNA was also found in epididymal mesenchyme of the human fetus, suggesting that this expression pattern could be conserved and a functional role could exist at least in mice and humans (35).
The spatial and temporal expression of Inhba exhibits a unique anterior-to-posterior gradient in the Wolffian duct mesenchyme. Expression of Inhba starts in the most anterior part of the Wolffian duct at E12.5 and as development proceeds, the expression domain gradually extends posteriorly. This gradient of Inhba expression corresponds to the degree of coiling in the epididymis. Initial expression of Inhba is restricted to the future caput epididymis where the most epithelial coiling occurs. As Inhba expression wanes along the anterior–posterior axis, the degree of coiling in the corpus and caudal epididymis decreases. It is interesting to note that Inhba expression is also found at later stages in the posterior Wolffian duct (future vas deferens) where no coiling is found. We therefore speculate that the function of Inhba on epididymal coiling is to prime the Woffian duct epithelium. Early exposure to INHBA or its protein product activin A predisposes the anterior Wolffian duct epithelium to obtain the coiling phenotype. On the other hand, in the posterior Wolffian duct where Inhba expression appears late, the Wolffian duct epithelium is unable to acquire the ability to coil.
Parameters of Androgen Action Are Not Affected in the Absence of Inhba.
Development of the male reproductive organs, including the epididymis, is thought to be controlled mainly by testosterone and its derivatives. In utero disruption of the androgen pathway led to various reproductive defects in male offspring. For example, loss of epididymal coiling was reported in rat embryos exposed to Di(n-Butyl) phthalate, which caused a decrease in androgen biosynthesis (36). Similarly, embryos exposed to linuron, a weak AR antagonist, also exhibited decreased epididymal coiling (37). Based on these observations, we initially speculated that the loss of epididymal coiling in the Inhba−/− mutant was the result of defects in systemic androgen production, instead of a direct local effect of Inhba. However, we found the level of testosterone per testis and parameters for androgen synthesis and action (Leydig cell differentiation, testis descent, anogenital distance, and AR expression in the mesenchyme) were normal in Inhba−/− embryos, therefore ruling out the possibility that androgen deficiency was the cause of lack of epididymal coiling. Androgens are known to maintain and stabilize the Wolffian duct but their involvement in the regional specification of the Wolffian duct is questionable as the entire Wolffian duct is exposed to androgens. It has been proposed that intrinsic factors such as homeobox genes or locally produced morphogens could be responsible for the transformation of the straight Wolffian duct into various segments of the male reproductive tract (38). Our results provide genetic evidence that Inhba serves as a paracrine regulator specific for the epididymal transformation as vas deferens and other parts of the male reproductive tract remain normal in Inhba−/− embryos. The undisturbed androgen parameters in the Inhba−/− embryos further indicate that androgens alone are not sufficient to facilitate epididymal coiling, supporting the hypothesis proposed by Cunha and Young (42).
In addition to the epididymal phenotypes, testis cord formation was affected in the Inhba null animals. This finding led to a concern that the loss of epididymal coiling in Inhba−/− male could be secondary to the testis phenotypes. To test this possibility, we examined markers for the differentiation of Sertoli cells and Leydig cells and found that these markers were properly expressed in the Inhba mutants despite the morphological defects. We also found that Inhba was essential for Sertoli cell proliferation but not their differentiation (data not shown). We therefore conclude that the epididymal phenotypes in the Inhba embryos resulted from the loss of local Inhba functions, not a secondary effect of the testis phenotypes or suboptimal testosterone production. Existing literature also provides evidence that epididymis formation can occur in the absence of testes. First, Alfred Jost (1, 2) in the 1940s and 1950s demonstrated that the Wolffian duct differentiated into various parts of the male reproductive tract in ovariectomized female rabbit embryos implanted with testosterone capsules. Second, Tsuji et al. (40) demonstrated that when the Wolffian duct was cultured without the testis in a serum-free medium with testosterone supplement proper epididymal coiling occurred. This evidence further strengthens the idea that testis-derived androgen and local signaling factors in the epididymis are sufficient to induce epididymal coiling.
Inhba Is Responsible for the Up-Regulation of AR in the Epididymal Epithelium.
It is worth noting that AR expression in the epididymal epithelium of Inhba−/− embryo was not up-regulated as seen in the wild-type embryos. During normal development, AR expression first appears in the mesenchyme and later in the epithelium of the Wolffian duct. It was proposed that the Wolffian duct mesenchyme produces a diffusible factor to stimulate AR expression in the epithelium (41). Our finding on the reduction of AR expression in the Inhba−/− epididymal epithelium provides evidence that INHBA or its product activin A is a candidate for this elusive diffusible factor. The consequence of loss of AR in the Inhba−/− epithelium is not clear. Based on the recombinant experiments using prostate, seminal vesicle, and mammary gland, AR expression in the epithelium is not essential for epithelial morphogenesis, remodeling, and proliferation in these tissues (11, 12, 42, 43). Therefore, we conclude that loss of AR expression in the epithelium does not account for the loss of coiling in the epididymis.
The convoluted organization of the epididymis is essential for sperm protection, maturation, concentration, and storage in the adult. We speculate that the Inhba−/− epididymis will not be functional in the adult. Unfortunately, we were not able to confirm this as the Inhba−/− embryos die around birth.
Initial Expression of Inhba in the Wolffian Duct Is Androgen-Independent.
Another important discovery of this research is that the initial Inhba expression in the anterior Wolffian duct does not depend on androgens. Inhba expression was present at E11.5 and E12.5, before the appearance of androgen-producing fetal Leydig cells. In addition, Inhba expression was found in the female Wolffian duct where no androgens were produced. However, the maintenance of Inhba expression seems to require the presence of a testis or testosterone as Inhba expression was decreased in the Wolffian duct when the testis was removed in culture. We also found that testosterone supplementation partially maintained the expression domain of Inhba in the absence of the testis. It remains to be determined whether the effect of testosterone on Inhba expression is mediated directly via transcriptional activation of the Inhba gene or indirectly by maintaining survival of the mesenchyme.
In summary, we have identified Inhba as a mesenchyme-derived gene that facilitates coiling of the epididymal epithelium. The Inhba knockout embryos exhibit normal androgen parameters, indicating that regulation of epididymal coiling by Inhba is not directly controlled by androgens. This is further supported by the fact that initial expression of Inhba does not require the presence of androgens. Defects in Wolffian duct patterning were also reported in homeobox gene mutations such as Hoxa-10 and Hoxa–11. Hoxa-10 and Hoxa–11 are expressed in a pattern opposite to Inhba, as the expression domain of these Hoxa genes was restricted to the posterior Wolffian duct. The epididymal coiling was normal in both Hoxa-10 and Hoxa-11 knockout embryos but ectopic coiling was observed in the vas deferens that derive from the posterior Wolffian duct (44, 45), suggesting that these Hoxa genes are critical for maintaining the identity of the vas deferens. It will be of particular interest in the future to investigate how INHBA interacts with these HOXA transcription factors to define the boundary between the epididymis and vas deferens. Furthermore, Fgf10 has been implicated as a mesenchyme-derived factor that controls Wolffian duct differentiation (46, 47). Interestingly in the Fgf10 knockout male epididymal coiling was not affected; instead, posterior Wolffian duct derivatives such as prostate and seminal vesicle were malformed (47). These two findings suggest that Inhba and Fgf10 are regionally specific mesenchymal genes that control anterior-to-posterior morphogenesis of the Wolffian duct.
Materials and Methods
Animals.
Inhba+/− mice were crossed to produce Inhba−/− animals (48). Genotypes were determined by PCR with the following primers: 5′-ACAGAACCAGGACCAAAGTCACCA-3′ and 5′-TCCAGTCATTCCAGCCAATGTCCT-3′ to detect the wild-type allele and 5′-GGACCTCTCGAAGTGTTGGATAC-3′ and 5′-CTTGCGCTCATCTTAGGCTT-3′ to detect the HPRT marker for the knockout allele. Timed matings were produced by housing female mice with males overnight and checking for vaginal plugs the next morning (E0.5 = noon of the day when a vaginal plug was found). Fetal tissue was collected at E13.5, E15.5, E17.5, and E19.5. If parturition occurred on E19.5, the newborn mice were used for tissue collection. All procedures described were reviewed and approved by the Institutional Animal Care and Use Committee and performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Testosterone RIA.
Testes were collected from newborn mice, and each epididymis was removed. Each testis was assayed individually for both testosterone and protein. Testosterone levels were determined in individually homogenized testes with a previously validated RIA (49). Testosterone concentrations (pg/mg protein) per testis were determined by using the RIAEIA Parallelism Program with Hot Recovery written by M.-C. J. Wu (Taiwan Livestock Research Institute, Hsinhua, Taiwan) (50).
Whole-Mount in Situ Hybridization.
Tissues were fixed overnight with 4% paraformaldehyde in PBS at 4°C and processed according to standard nonradioisotopic procedures using digoxigenin-labeled RNA probes (51). The RNA probes and their optimal hybridization temperatures were: Inhba (65°C), Pax2 (62.5°C), and P450 SCC (65°C).
Periodic Acid/Schiff Staining.
Tissues were immersion fixed in 4% glutaldehyde, dehydrated through an ethanol gradient, and embedded in glycol methacrylate. Blocks were cut into 2.5-μm sections, and every fifth section was collected on a glass slide. Sections were stained by using the periodic acid/Schiff reagent for 45 min and counterstained with hematoxylin for 35 min.
Immunocytochemistry and TUNEL Assay.
Tissues were fixed in 4% paraformaldehyde in PBS at 4°C overnight and dehydrated through a sucrose gradient. Tissues were then embedded in Histoprep frozen tissue embedding media (Fisher, Waltham, MA) and cryosectioned to 8 μm thick and dried on polylysine-treated slides. Slides were either processed for immunocytochemistry or apoptosis assay. For immunocytochemistry, endogenous hydrogen peroxidase was quenched for 30 min at room temperature in 0.6% H2O2 in methanol followed by a 10-min running tap water wash. Sections were next treated with a microwave-heated antigen retrieval solution (0.1 M citrate, pH 6) for 10 min (Ki67) or 20 min (AR and phospho-SMAD 2/3). After cooling, sections were washed 10 min in running tap water and then incubated with 10% donkey serum in PBS for 1 h followed by overnight incubation with primary antibody diluted in PBS at 4°C. Primary antibodies used included phospho-SMAD 2/3 (1:250; Cell Signaling, Beverly, MA), Ki67 (1:500; BD Pharmingen, Franklin Lakes, NJ), and AR (1:500; generously provided by Gail Prins, University of Illinois, Chicago, IL). Sections were washed with PBS (three times at 15 min each) and then incubated with the secondary antibody diluted in 5% donkey serum in PBS for 1 h at room temperature. Secondary antibodies used included biotinylated donkey anti-mouse (1:200; Jackson, West Grove, PA) for Ki67 and biotinylated donkey anti-rabbit (1:200; Jackson) for AR and phospho-SMAD 2/3. Sections were washed with PBS, and signal was developed by using the TSA Fluorescein System kit (PerkinElmer, Wellesley, MA) according to the manufacturer's instructions and modification (39). Sections were counterstained with DAPI containing Vectashield mounting medium (Vector, Burlingame, CA). Cryosectioned slides designated for measuring apoptosis were TUNEL-stained by using the in situ cell death detection kit (Roche, Indianapolis, IN) following the manufacturer's instructions.
Evaluation of Epithelial Proliferation and Apoptosis.
Ki67 immunocytochemistry and TUNEL assay were used to label proliferating and apoptotic cells, respectively. For each tissue (wild type and Inhba null), serial sections, amounting to at least 10 sections, 24 μm apart between each section, were pictured, and the caput, corpus, and caudal portions of the Wolffian duct were identified. The total cell number (DAPI staining for individual nucleus) and total proliferating cells (Ki67-positive) or apoptotic cells (TUNEL-positive) were obtained for each epithelial portion. The percentage proliferation or apoptosis was calculated as the fraction of Ki67- or TUNEL-positive cells over the total number of nuclei.
Explant Culture.
Mesonephroi with testes attached were isolated from E13.5 embryos and cultured for 48 h on a 1.5% agar block in DMEM supplemented with 10% charcoal-stripped FCS and 50 μg/ml ampicillin at 37°C with 5% CO2/95% air. For androgen-dependency studies, testes were removed and only mesonephroi were cultured in the presence of testosterone (10−7 M) for 48 h. An equivalent volume of methanol (solvent for testosterone) was added to control cultures. Changes of fresh medium with or without testosterone was performed every 24 h.
Statistical Analysis.
Data were tested for normality followed by two-way ANOVA, and the significance was decided if P < 0.01.
Supplementary Material
Acknowledgments
We thank Dr. Marty Matzuk (Baylor College of Medicine, Houston, TX) for the Inhba+/− mouse, Dr. Gail Prins for the AR antibody, Dr. Janice Bahr for assistance with testosterone measurement, Dr. Barry Hinton for critical comments on the manuscript, and members of H.H.-C.Y.'s laboratory for support. This work was supported by a Howard Hughes Undergraduate Research Fellowship (to J.T.), a National Institute of Environmental Health Sciences Toxicology Training Fellowship (to A.J.), a Environmental Council Toxicology Scholar Award (to A.J.), the March of Dimes Birth Defects Foundation (Basil O'Conner Starter Scholar Research Award 5-FY04-35; to H.H.-C.Y.), and National Institutes of Health Grant HD46861 (to H.H.-C.Y.).
Abbreviations
- AR
androgen receptor
- En
embryonic day
- Inhba
inhibin beta A.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703445104/DC1.
References
- 1.Jost A. Arch Anat Microsc Morphol Exp. 1947;36:271–315. [Google Scholar]
- 2.Jost A. Recent Prog Horm Res. 1953;8:379–418. [Google Scholar]
- 3.Drews U. Anat Embryol (Berl) 1975;146:325–340. doi: 10.1007/BF00302178. [DOI] [PubMed] [Google Scholar]
- 4.Brown TR, Scherer PA, Chang YT, Migeon CJ, Ghirri P, Murono K, Zhou Z. Eur J Pediatr. 1993;152(Suppl 2):S62–S69. doi: 10.1007/BF02125442. [DOI] [PubMed] [Google Scholar]
- 5.Brown TR. J Androl. 1995;16:299–303. [PubMed] [Google Scholar]
- 6.Imperato-McGinley J, Sanchez RS, Spencer JR, Yee B, Vaughan ED. Endocrinology. 1992;131:1149–1156. doi: 10.1210/endo.131.3.1324152. [DOI] [PubMed] [Google Scholar]
- 7.Euling SY, Kimmel CA. Sci Total Environ. 2001;274:103–113. doi: 10.1016/s0048-9697(01)00736-7. [DOI] [PubMed] [Google Scholar]
- 8.Cooke PS, Young P, Cunha GR. Endocrinology. 1991;128:2867–2873. doi: 10.1210/endo-128-6-2867. [DOI] [PubMed] [Google Scholar]
- 9.Huhtaniemi I. Eur J Endocrinol. 1994;130:25–31. doi: 10.1530/eje.0.1300025. [DOI] [PubMed] [Google Scholar]
- 10.Higgins SJ, Young P, Cunha GR. Development (Cambridge, UK) 1989;106:235–250. doi: 10.1242/dev.106.2.235. [DOI] [PubMed] [Google Scholar]
- 11.Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 12.Cunha GR, Lung B. J Exp Zool. 1978;205:181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- 13.Sugimura Y, Cunha GR, Bigsby RM. Prostate. 1986;9:217–225. doi: 10.1002/pros.2990090302. [DOI] [PubMed] [Google Scholar]
- 14.Yao HH, Aardema J, Holthusen K. Biol Reprod. 2006;74:978–983. doi: 10.1095/biolreprod.105.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancilla B, Jarred RA, Wang H, Mellor SL, Cunha GR, Risbridger GP. Dev Biol. 2001;237:145–158. doi: 10.1006/dbio.2001.0364. [DOI] [PubMed] [Google Scholar]
- 16.Ball EM, Risbridger GP. Dev Biol. 2001;238:1–12. doi: 10.1006/dbio.2001.0399. [DOI] [PubMed] [Google Scholar]
- 17.Davies JA, Fisher CE. Exp Nephrol. 2002;10:102–113. doi: 10.1159/000049905. [DOI] [PubMed] [Google Scholar]
- 18.Davies J. J Anat. 2001;198:257–264. doi: 10.1046/j.1469-7580.2000.19830257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang PT, McMahon AP. Trends Cell Biol. 2003;13:86–91. doi: 10.1016/s0962-8924(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 20.Hovey RC, Trott JF. Adv Exp Med Biol. 2004;554:219–228. doi: 10.1007/978-1-4757-4242-8_19. [DOI] [PubMed] [Google Scholar]
- 21.Cunha GR, Cooke PS, Kurita T. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 22.Gupta C, Bentlejewski CA. Biol Reprod. 1992;47:1151–1160. doi: 10.1095/biolreprod47.6.1151. [DOI] [PubMed] [Google Scholar]
- 23.Gupta C, Singh M. Endocrinology. 1996;137:705–711. doi: 10.1210/endo.137.2.8593821. [DOI] [PubMed] [Google Scholar]
- 24.Alarid ET, Rubin JS, Young P, Chedid M, Ron D, Aaronson SA, Cunha GR. Proc Natl Acad Sci USA. 1994;91:1074–1078. doi: 10.1073/pnas.91.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo MA, Giustizieri ML, Favale A, Fantini MC, Campagnolo L, Konda D, Germano F, Farini D, Manna C, Siracusa G. Biol Reprod. 1999;61:1123–1132. doi: 10.1095/biolreprod61.4.1123. [DOI] [PubMed] [Google Scholar]
- 26.Alarid ET, Cunha GR, Young P, Nicoll CS. Endocrinology. 1991;129:2148–2154. doi: 10.1210/endo-129-4-2148. [DOI] [PubMed] [Google Scholar]
- 27.de Kretser DM, Hedger MP, Loveland KL, Phillips DJ. Hum Reprod Update. 2002;8:529–541. doi: 10.1093/humupd/8.6.529. [DOI] [PubMed] [Google Scholar]
- 28.Tong S, Wallace EM, Burger HG. Clin Endocrinol (Oxford) 2003;58:115–127. doi: 10.1046/j.1365-2265.2003.01686.x. [DOI] [PubMed] [Google Scholar]
- 29.Itoh S, Itoh F, Goumans MJ, Ten Dijke P. Eur J Biochem. 2000;267:6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- 30.Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Genes Cells. 2002;7:1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 31.Abe Y, Minegishi T, Leung PC. Growth Factors. 2004;22:105–110. doi: 10.1080/08977190410001704688. [DOI] [PubMed] [Google Scholar]
- 32.Gray PC, Greenwald J, Blount AL, Kunitake KS, Donaldson CJ, Choe S, Vale W. J Biol Chem. 2000;275:3206–3212. doi: 10.1074/jbc.275.5.3206. [DOI] [PubMed] [Google Scholar]
- 33.Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. Nature. 2000;404:411–414. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- 34.Esquela AF, Lee SJ. Dev Biol. 2003;257:356–370. doi: 10.1016/s0012-1606(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 35.Roberts VJ. Endocrine. 1997;6:85–90. doi: 10.1007/BF02738807. [DOI] [PubMed] [Google Scholar]
- 36.Bowman CJ, Turner KJ, Sar M, Barlow NJ, Gaido KW, Foster PM. Toxicol Sci. 2005;86:161–174. doi: 10.1093/toxsci/kfi172. [DOI] [PubMed] [Google Scholar]
- 37.Turner KJ, McIntyre BS, Phillips SL, Barlow NJ, Bowman CJ, Foster PM. Toxicol Sci. 2003;74:114–128. doi: 10.1093/toxsci/kfg096. [DOI] [PubMed] [Google Scholar]
- 38.Bomgardner D, Hinton BT, Turner TT. J Androl. 2001;22:527–531. [PubMed] [Google Scholar]
- 39.Nie R, Zhou Q, Jassim E, Saunders PT, Hess RA. Biol Reprod. 2002;66:1161–1168. doi: 10.1095/biolreprod66.4.1161. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji M, Shima H, Cunha GR. Endocrinology. 1991;128:1805–1811. doi: 10.1210/endo-128-4-1805. [DOI] [PubMed] [Google Scholar]
- 41.Cunha GR, Reese BA, Sekkingstad M. Endocrinology. 1980;107:1767–1770. doi: 10.1210/endo-107-6-1767. [DOI] [PubMed] [Google Scholar]
- 42.Cunha GR, Young P. Endocrinology. 1991;128:3293–3298. doi: 10.1210/endo-128-6-3293. [DOI] [PubMed] [Google Scholar]
- 43.Durnberger H, Kratochwil K. Cell. 1980;19:465–471. doi: 10.1016/0092-8674(80)90521-8. [DOI] [PubMed] [Google Scholar]
- 44.Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Development (Cambridge, UK) 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K, Potter SS. Development (Cambridge, UK) 1995;121:1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- 46.Thomson AA, Cunha GR. Development (Cambridge, UK) 1999;126:3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- 47.Donjacour AA, Thomson AA, Cunha GR. Dev Biol. 2003;261:39–54. doi: 10.1016/s0012-1606(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 48.Matzuk MM, Kumar TR, Bradley A. Nature. 1995;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- 49.Palmer SS, Nelson RA, Ramsay MA, Stirling I, Bahr JM. Biol Reprod. 1988;38:1044–1050. doi: 10.1095/biolreprod38.5.1044. [DOI] [PubMed] [Google Scholar]
- 50.Bahr JM, Wang SC, Huang MY, Calvo FO. Biol Reprod. 1983;29:326–334. doi: 10.1095/biolreprod29.2.326. [DOI] [PubMed] [Google Scholar]
- 51.Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.