Abstract
Wnt signaling is required for the maintenance of intestinal stem cells and self-renewal of the intestinal epithelium. Intestinal cancers are frequently associated with mutations that activate the Wnt pathway. The role of Wnt signaling on differentiation of lineage-specific precursors in the intestine is not well characterized. Here, we show that specification of enteroendocrine but not Paneth cells occurs independently of Wnt signals by conditional deletion of β-catenin in immature cells expressing the transcription factor, neurogenin 3. In addition, we determined whether neurogenin 3-expressing cells respond to abnormal Wnt signaling. Activation of the Wnt pathway by conditionally deleting exon 3 of the β-catenin gene at an early stage of enteroendocrine cell differentiation induced small-intestinal adenomas expressing serotonin, a feature not previously described in other tumors induced by Wnt in mice. In contrast, excision of exon 3 of β-catenin at a later stage of enteroendocrine differentiation did not produce tumors. These results provide direct evidence that some intestinal lineages are specified independently of the Wnt pathway and may lead to a better understanding of the spectrum of neuroendocrine differentiation frequently seen in human gastrointestinal cancer.
Keywords: carcinoid, enteroendocrine cell, neurogenin 3, Paneth cell, Wnt pathway
Activation of canonical Wnt signaling results in a series of events that stabilize β-catenin, with resultant activation of Wnt target gene transcription (1). Generalized disruption of Wnt signaling in the intestine resulted in widespread loss of crypts with the absence of cell proliferation in the small intestine, indicating an essential role for this pathway in the maintenance of intestinal stem cells and their dividing descendents. Enteroendocrine cells failed to develop in both TCF4-null and villin-Dkk1 mice, whereas goblet cells were absent in villin-Dkk1 but not TCF4−/− animals (2, 3). The absence of enteroendocrine cells with generalized Wnt inactivation in the intestine may indicate that this lineage requires Wnt signals for differentiation. Alternatively, depletion of intestinal stem cells may preclude the ongoing replacement of enteroendocrine cells which turnover every 4–5 days rather than directly requiring Wnt signals for their specification.
Intestinal neoplasms, including colorectal cancer in humans, are frequently associated with excessive Wnt signaling. APCmin mice with a mutant APC allele develop adenomatous polyps in the intestine, as do mice expressing a mutant β-catenin allele lacking exon 3. In both cases, reduced targeting of β-catenin for degradation leads to redistribution of β-catenin to the nucleus with activation of Wnt target genes (4, 5).
Two basic helix–loop–helix (bHLH) proteins regulated by the Notch signaling pathway (6, 7), neurogenin 3 (NGN3) and NeuroD1, have an established role in the specification and differentiation of enteroendocrine cells. Mice lacking Ngn3 develop few enteroendocrine cells, indicating an essential role for their specification (8, 9). Ngn3 is transiently expressed in immature cells to initiate enteroendocrine differentiation and induces expression of NeuroD1 (10). We previously showed that NeuroD1 is expressed in most enteroendocrine cells and induces cell-cycle arrest during later stages of enteroendocrine differentiation (11). Thus, expression of Ngn3 and NeuroD represent distinct stages in the differentiation of enteroendocrine cells from intestinal secretory progenitor cells in intestinal crypts.
Currently, it is not known whether cells committed to become enteroendocrine cells depend on Wnt signals for further differentiation or whether they retain the capacity to respond to Wnt. In the present work, we found that early precursors to enteroendocrine cells respond to abnormal Wnt signaling to develop serotonin-expressing adenomas of the small intestine, whereas later precursors do not. In addition, our results indicate that Wnt signaling is required for Paneth cells to differentiate but not for specification of enteroendocrine cells.
Results and Discussion
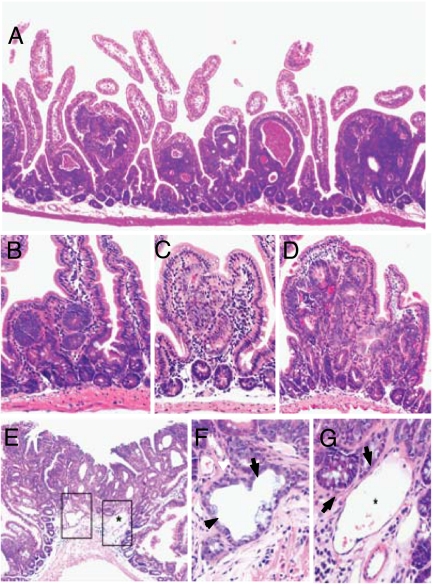
To determine whether enteroendocrine progenitor cells respond to abnormal Wnt signaling, we conditionally deleted exon 3 of a mutant β-catenin allele in CatnbFlox(ex3)/+ mice (4) expressing Cre under control of the Ngn3 gene (12) to generate a stabilized β-catenin protein. Ngn3-Cre:CatnbFlox(ex3)/+ mice were born with the expected frequency. After 4 weeks of age, we observed increasing numbers of adenomatous polyps throughout the small intestine (Fig. 1A) but not in other Ngn3-expressing gastrointestinal organs, including colon, stomach, and pancreas, consistent with earlier work suggesting that the small intestine is the predominant organ in the GI tract targeted by exon 3 deletions of β-catenin where mice harboring up to 3,000 adenomas fail to develop colonic tumors (4, 5). Polyps appeared to originate from aberrant crypts (Fig. 1 B and C) and in some cases displayed high-grade dysplasia with nuclear pseudostratification, increased mitotic figures, and mucosal ulceration consistent with intraepithelial carcinoma (Fig. 1D). At 20 weeks of age, we observed 246 ± 40 polyps per mouse (Fig. 3A). Most Ngn3-Cre:CatnbFlox(ex3)/+ mice developed a single or occasionally two bulky invasive adenocarcinomas with tumor glands invading beyond the muscularis mucosa into the submucosal space to the muscularis propria (Fig. 1 E–G) and died without distant metastasis by 6–9 months of age (Fig. 3B). The very low frequency of invasive neoplasia is consistent with earlier observations following conditional deletion of exon 3 of the β-catenin gene throughout the intestine (4, 5).
Fig. 1.
Small-intestinal neoplasms in Ngn3-Cre:CatnbFlox(ex3)/+ mice. (A–D) Polypoid neoplasms in a 2-month-old Ngn3-Cre:CatnbFlox(ex3)/+ mouse (hematoxylin/eosin staining). (A) Low-power view showing polypoid neoplasms in the small intestine. (B) Aberrant crypt focus within the lamina propria. (C) Dysplastic aberrant crypt within two enlarged villi. (D) Early intramucosal carcinoma showing multiple aberrant crypts with glandular fusion and severe cytological atypia. (E) A large carcinoma with superficial ulceration and neoplastic glands invading beyond the muscularis mucosa. The asterisk shows a dilated submucosal vessel. (F) High-magnification view showing neoplastic glands (arrows) invading the submucosa beyond the muscularis mucosae and close to the muscularis propria (lower right corner). (G) Some neoplastic glands (arrows) are seen close to a small vessel wall (asterisk).
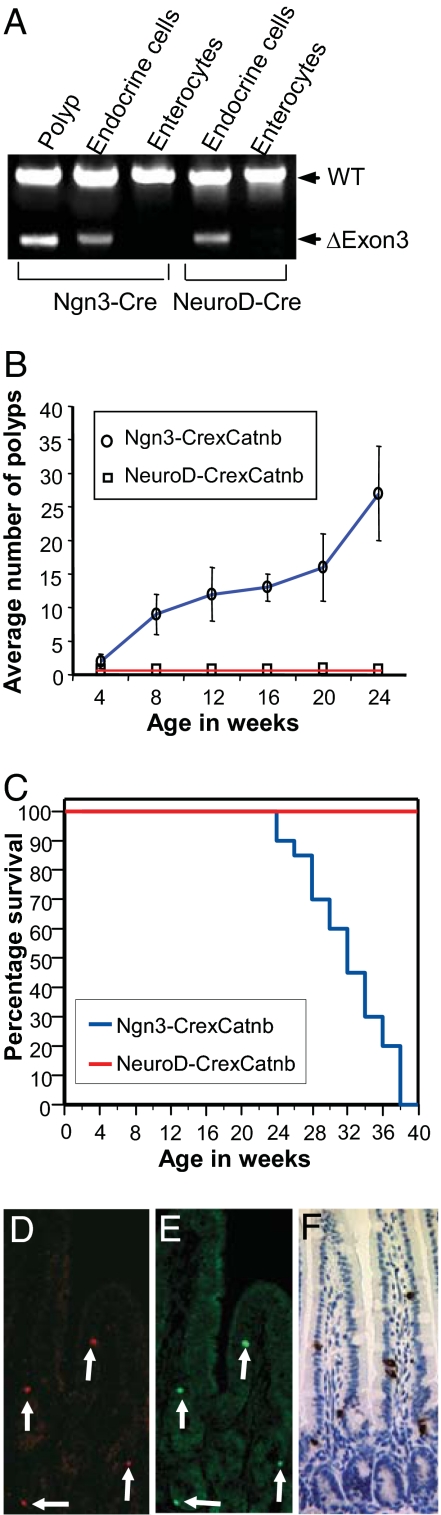
Fig. 3.
Absence of neoplasia in NeuroD-Cre:CatnbFlox(ex3)/+ mice. (A) Genotyping for excision of exon 3 of the β-catenin gene by amplification of DNA from chromogranin A-stained enteroendocrine cells or enterocytes isolated by laser microdissection from the small intestine of Ngn3-Cre:CatnbFlox(ex3)/+ and NeuroD-Cre:CatnbFlox(ex3)/+ mice. Arrows denote the positions of the wild-type and exon 3 deleted alleles. DNA was amplified 45–54 cycles. (B) Ngn3-Cre:CatnbFlox(ex3)/+ mice (circles) accumulate neoplasms with increasing age, whereas NeuroD-Cre:CatnbFlox(ex3)/+ mice (squares) fail to develop polyps at any age. For each time point, polyps were counted in three to four animals and results were expressed as the number of polyps ±SEM per cm of intestinal length. (C) Survival curves for Ngn3-Cre:CatnbFlox(ex3)/+ (blue) and NeuroD-Cre:CatnbFlox(ex3)/+ (red) mice showing the percentage of mice remaining alive at 2-week intervals. The analysis included 20 animals for each genotype. Most Ngn3-Cre:CatnbFlox(ex3)/+ mice died by the age of 9 months in contrast to NeuroD-Cre:CatnbFlox(ex3)/+ mice. (D and E) Colocalization of Cre (C) and NeuroD (D) by double immunofluorescence in the small intestine of NeuroD-Cre mice. (F) NeuroD-Cre:CatnbFlox(ex3)/+ mouse display normal intestinal morphology and normal enteroendocrine cells immunostained for chromogranin A.
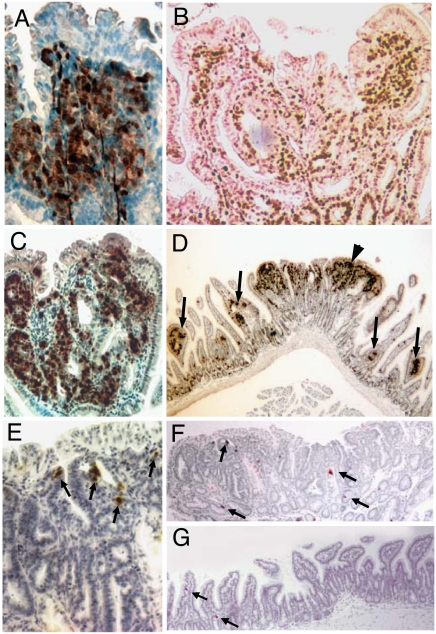
We stained intestinal sections from Ngn3-Cre:CatnbFlox(ex3)/+ mice for the general marker of neuroendocrine (NE) differentiation, PGP 9.5 (13) (Fig. 2A) to determine whether the adenomas were of neuroendocrine origin. Most adenomas had clusters of cells staining for this marker. Cells within the adenomas stained for Ki67, indicating that they were actively proliferating (Fig. 2B). In addition, >80% of the adenomas stained for the specific NE marker, serotonin (Fig. 2 C and D). Although we identified rare single cells staining for the general NE marker chromogranin A, immunohistochemistry for other specific NE markers including the hormones secretin, cholecystokinin, and somatostatin was negative. Neuroendocrine differentiation has not been previously described in intestinal neoplasms associated with abnormal Wnt signaling in mice, including mice with generalized intestinal activation of the Wnt pathway. In contrast to Ngn3-Cre:CatnbFlox(ex3)/+ mice, Apcmin mice developed adenomas with infrequent serotonin-expressing cells, which usually appeared as single cells and rarely as two to three cells in close proximity but never in larger clusters (Fig. 2 E–G). The absence of neuroendocrine features in other Wnt-related intestinal tumors suggests that the serotonin-expressing adenomas probably arose from recombination in Ngn3+ cells that were precursors to enteroendocrine cells. Within the limits of the assays, amplification of DNA extracted from enteroendocrine cells and enterocytes isolated by laser microdissection revealed that recombination at the β-catenin locus occurred much less frequently in enterocytes compared with enteroendocrine cells, further suggesting that the observed tumors arose from the enteroendocrine lineage (Fig. 3A).
Fig. 2.
Neuroendocrine features in small-intestinal tumors in Ngn3-Cre:CatnbFlox(ex3)/+ mice. (A) Small-intestinal neoplasm from an Ngn3-Cre:CatnbFlox(ex3)/+ mouse immunostained for general neuroendocrine marker, PGP9.5. (B) Polypoid neoplasm stained for Ki67. (C and D) Small-intestinal neoplasms in Ngn3-Cre:CatnbFlox(ex3)/+ mice showing expression of serotonin (brown) in high (C) and low (D) magnification. Arrows denote small nascent polyps. Arrowhead, large polyp. (E–G) Small intestine from an Apcmin mouse with small adenoma (G) and large adenomas at high (E) and low (F) magnification immunostained for serotonin (brown). Arrows denote serotonin-stained cells.
Abnormal Wnt signaling has been reported to occur in up to 80% of human gastric, intestinal, and rectal carcinoid tumors that are also classified as well differentiated NE tumors (14). In this study, nearly 40% of tumors had a single point mutation in the third exon of β-catenin with 26 of 29 of these tumors containing Ser-Ala mutation in codon 37, a residue that is normally phosphorylated by GSK-3β. Another 40% of human tumors showed redistribution of β-catenin, suggesting excessive Wnt signaling without defined mutations in either β-catenin or the APC gene (14). Human carcinoids show relatively uniform expression of NE markers as do other less frequent small-intestinal endocrine tumors (15). In the present work, the absence of serotonin-expressing neoplasms in the body of the stomach, a frequent site for the occurrence of neuroendocrine tumors in rodents and humans, was not surprising, because most enteroendocrine cells in this region of the stomach do not arise from Ngn3+ cells (12). Although the tumors reported here show some similarity to carcinoids, many tumor cells did not stain for NE markers and may be related to “mixed exocrine-endocrine carcinoma” (MEEC), which contain significant endocrine and nonendocrine components (16).
Genotyping of normal enteroendocrine cells in Ngn3-Cre:CatnbFlox(ex3)/+ mice revealed recombination at exon 3 of β-catenin, suggesting that excision of exon 3 preceded polyp formation (Fig. 3A). The delayed appearance of polyps suggests that development of neoplasia may require ongoing exposure to excessive Wnt signaling. The absence of tumors in mice with biallelic deletion of APC in the intestine also suggests the importance of sustained abnormal Wnt signaling for tumorigenesis. Despite replacement of the intestinal epithelium with proliferating crypt cells, these mice fail to develop neoplasms, presumably because of their short lifespan (17).
To determine whether enteroendocrine precursors retain the ability to respond to abnormal Wnt signals as they differentiate, we generated mice expressing Cre recombinase under control of 115 kb of 5′ sequence and 78 kb of 3′ sequence of the NeuroD gene, a bHLH transcription factor induced by Ngn3 in enteroendocrine cells. Thus, the onset of NeuroD expression represents a later stage in the differentiation of enteroendocrine cells than expression of Ngn3. Expression of the Cre transgene appeared identical to the endogenous NeuroD gene because it was restricted to NeuroD+ cells in the small intestine with all NeuroD+ cells showing expression of Cre (Fig. 3 C and D). We crossed the NeuroD-Cre mice with CatnbFlox(ex3)/+ mice to initiate excision of the third exon of the β-catenin gene with the onset of NeuroD expression, later in differentiation compared with the Ngn3-Cre:CatnbFlox(ex3)/+ mice.
NeuroD-Cre:CatnbFlox(ex3)/+ mice remained healthy and free of polyps with normal intestinal morphology and enteroendocrine cells at 1 year of age (Fig. 3 A, B, and E). Amplification of the β-catenin gene from enteroendocrine cells isolated from NeuroD-Cre:CatnbFlox(ex3)/+ mice by laser microdissection showed excision of exon 3 from the floxed β-catenin allele but not the wild-type allele as expected. Excision occurred specifically in enteroendocrine cells but not in enterocytes, indicating that the failure to develop intestinal neoplasms did not result from the absence of recombination in enteroendocrine cells (Fig. 3A). We have previously shown that expression of NeuroD is important for differentiating enteroendocrine cells to undergo cell-cycle arrest (11). Thus, initiation of NeuroD expression may represent a stage when differentiating enteroendocrine cells permanently exit the cell cycle and lose their response to Wnt signals.
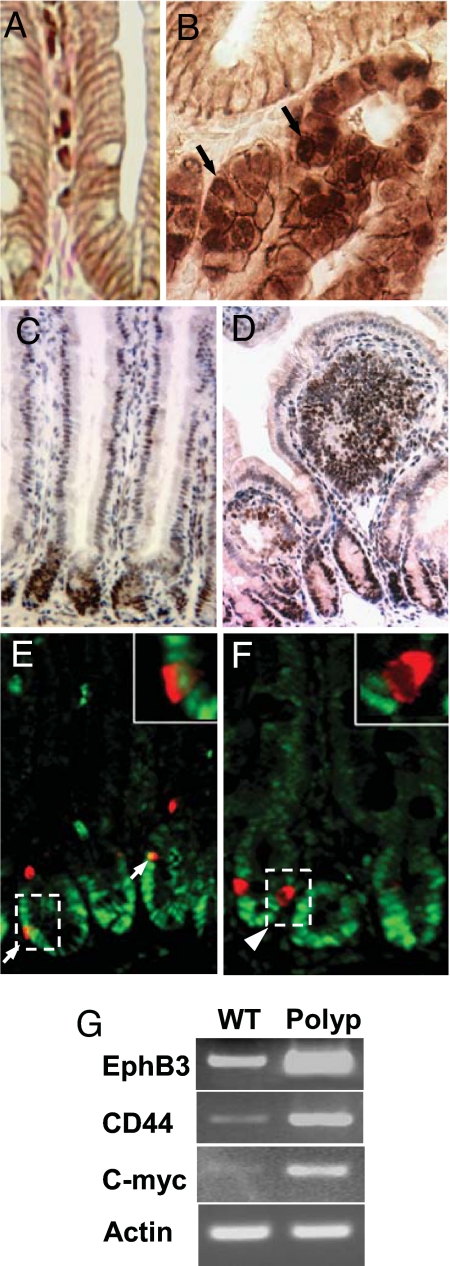
Neoplasms in Ngn3-Cre:CatnbFlox(ex3)/+ mice showed nuclear and cytoplasmic β-catenin staining indicative of active Wnt signaling, versus the predominant localization at the cell membrane in adherens junctions of normal mice (Fig. 4 A and B). In normal mice, expression of the Wnt target gene, c-Myc, was confined to cells in the lower crypt (Fig. 4C). Dysplastic epithelial cells invading the lamina propria of polyps showed strong nuclear staining for c-Myc, consistent with excessive Wnt signaling (Fig. 4D). We examined polyps for increased expression of two additional Wnt target genes, EphrinB3 and CD44, by semiquantitative RT-PCR. Polyps showed increased expression of both of these genes and of c-myc compared with adjacent normal epithelium (Fig. 4G).
Fig. 4.
Abnormal Wnt signaling in the intestine of Ngn3-Cre:CatnbFlox(ex3)/+ mice. (A and B) Intracellular localization of β-catenin. (A) Immunostaining for β-catenin at the cell membrane in a normal mouse. (B) Nuclear and cytoplasmic β-catenin immunostaining in a large adenoma. (C) Immunostaining for c-Myc in normal mice is restricted to crypts. (D) Polyp with widespread c-Myc staining. (E) Double immunofluorescent staining for c-Myc (green) and chromogranin A (red) shows some enteroendocrine cells in normal intestinal tissue of Ngn3-Cre:CatnbFlox(ex3)/+ mice that express c-Myc. Higher magnification (Inset) shows a single cell with red cytoplasmic staining for chromogranin A, colocalized with green nuclear c-Myc staining. (F) Absence of c-Myc staining in enteroendocrine cells of NeuroD-Cre:CatnbFlox(ex3)/+ mice. (G) Semiquantitative RT-PCR analysis comparing expression of Wnt target genes, c-myc, CD44, and EphrinB3, in polyps versus normal epithelium.
We also detected nuclear c-Myc staining in a fraction of enteroendocrine cells in normal appearing intestine of Ngn3-Cre:CatnbFlox(ex3)/+ mice (Fig. 4E) but not in NeuroD-Cre:CatnbFlox(ex3)/+ mice (Fig. 4F). Thus, excision of exon 3 of the β-catenin gene at this later stage of differentiation with the onset of NeuroD expression did not result in activation of the Wnt target gene, c-Myc. The failure to activate Wnt target genes in response to nuclear β-catenin has been described for mature T lymphocytes. Introduction of stabilized β-catenin into mature T lymphocytes did not result in Wnt target gene expression despite the formation of β-catenin–LEF1 complexes in the nucleus, in contrast to activation of Wnt target genes in the Jurkat T cell line (18). The underlying mechanism accounting for this differential response to β-catenin remains to be elucidated.
Generalized disruption of Wnt signaling in the intestine interferes with specification of all three secretory lineages (2, 3). The failure to develop secretory lineages in mice with the complete loss of Wnt signaling may result from depletion of stem cells rather than a lineage-specific requirement for this pathway. We conditionally deleted the β-catenin gene from Ngn3-expressing cells to determine whether Wnt signaling has a direct effect on the differentiation of enteroendocrine cells as opposed to its known role in stem-cell maintenance. We crossed Ngn3-Cre transgenic mice to animals with one null allele and one conditional β-catenin allele with loxP sites flanking exons 2–6 (19) into a R26R background to allow us to identify cells that expressed Cre on the basis of β-gal activity arising from the ROSA26 gene. The small intestine of Ngn3-Cre:R26R:CatnbFlox/− mice appeared normal, revealing normal crypt-villus architecture with proliferating cells confined to the crypt. The number of β-gal-expressing enteroendocrine cells in Ngn3-Cre:R26R:CatnbFlox/− mice was not significantly different from Ngn3-Cre:R26R mice, indicating that Wnt signaling was not required for their specification or terminal differentiation (Fig. 5A).
Fig. 5.
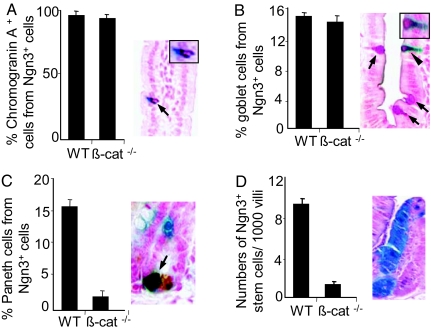
Specification of enteroendocrine and goblet cells but not Paneth cells occurs independently of Wnt signaling. (A–D) Effects of conditionally deleting β-catenin in intestinal epithelial lineages arising from Ngn3+ cells in a R26R background. The numbers of enteroendocrine, goblet, Paneth, and pluripotent cells arising from Ngn3+ cells stained for β-gal activity (blue) using X-gal histochemistry were counted. At least 500 cells were counted for each lineage. (A) Conditional deletion of the β-catenin gene in endocrine precursor cells expressing Ngn3 has no effect on enteroendocrine cell differentiation. Mice show normal numbers of chromogranin A-stained enteroendocrine cells (Left) and with normal distribution (arrow; Right). Higher magnification (Inset) shows a single cell with cytoplasmic staining for β-gal (blue) and chromogranin A (brown). (B) Number of β-gal-expressing cells showing PAS staining for mucin (red) was unaffected by the deletion of β-catenin (Right). Shown are examples of goblet cells double positive for PAS and β-gal (arrowhead) or PAS alone (arrows). Higher magnification (Inset) shows a single cell with cytoplasmic staining for PAS and β-gal. (C) Deletion of β-catenin resulted in an 85% reduction in the number of Paneth cells expressing β-gal activity (Right). Shown are examples of X-gal-positive (blue) and lysozyme-positive (brown) Paneth cells. (D) Descendants of pluripotent cells expressing Ngn3 appear as entire crypt-villus units that stain for β-gal activity (Right) and were reduced by 75% in number when β-catenin was deleted under control of the Ngn3 gene. Results were expressed as the percentage of goblet and Paneth cells expressing β-gal activity (B and C) or as the frequency of crypt-villus units per 1,000 showing expression of β-gal in all epithelial cells.
We previously showed that ≈5–10% of small-intestinal goblet cells, and 45% of Paneth cells arose from cells expressing Ngn3 by recombination based lineage analysis (12). To examine whether canonical Wnt signaling was required for specification of nonendocrine cells that arise from Ngn3+ cells, we examined the effect of deleting β-catenin in Ngn3+ cells in the small intestine of Ngn3-Cre:R26R:CatnbFlox/− mice on the number of β-gal expressing Paneth cells and goblet cells. The fraction of goblet cells expressing β-gal in Ngn3-Cre:R26R:CatnbFlox/− mice was identical to Ngn3-Cre:R26R mice, suggesting that this subpopulation of goblet cells did not depend on Wnt signaling for differentiation (Fig. 5B). However, we observed an 80% reduction in the fraction of Paneth cells expressing β-gal in Ngn3-Cre:R26R:CatnbFlox/− mice (Fig. 5C), suggesting that a major fraction of this lineage may depend on Wnt for specification. Recombination based lineage analysis also revealed that a very small fraction of pluripotent cells in the intestine expressed Ngn3, resulting in β-gal expression in all epithelial cells of single crypt-villus units at a frequency of <1%. We observed a 74% reduction in the frequency of crypt-villus units showing generalized β-gal expression in Ngn3-Cre:R26R:CatnbFlox/− mice, consistent with the established role of the canonical Wnt pathway in the maintenance of crypt stem cells (Fig. 5D).
The importance of Wnt signaling for Paneth cell specification is unresolved at present. Paneth cells in cryptless regions of the intestine of villin-Dkk1 mice were dramatically reduced in number. The loss of Paneth may have resulted from the relative absence of crypt stem cells rather than from a direct lineage-specific requirement (2, 3). Activation of the Wnt pathway by conditional APC deletion under control of the villin gene resulted in crypt expansion with increased numbers of Paneth cells, reduced goblet cell numbers, and a slight reduction in enteroendocrine cells, suggesting that Wnt signaling favors Paneth-cell differentiation at the expense of other secretory lineages (20).
The sparing of the enteroendocrine and goblet lineages after conditional deletion of β-catenin in the present work indicates that the loss of Paneth cells did not result from stem-cell depletion. Disruption of Wnt signaling by conditional deletion of Frizzled5 (Fz5) in keratin 19-expressing cells resulted in mispositioning of Paneth cells in the intestine, leading to the suggestion that Wnt signaling was required for maturation of Paneth cells but not for their specification (21, 22). However, multiple Frizzled family proteins, including Frizzled 5, Frizzled 6, and Frizzled 7, were identified at the crypt base overlapping the location of Paneth cells and their precursors (23). Thus, deletion of Frizzled 5 may only result in a partial loss of Wnt signaling and is consistent with mispositioning of Paneth cells in parts of the intestine of villin-Dkk1 mice with normal appearing crypts. We never observed mispositioning of Paneth cells to villi in Ngn3-Cre:R26R:CatnbFlox/− mice, suggesting that Paneth cells were not specified with complete disruption of the canonical Wnt pathway. Thus, specification, differentiation, and maturation of Paneth cells may require different components of the Wnt pathway.
The absence of goblet, Paneth, and enteroendocrine cells in mice lacking the bHLH protein MATH1 may indicate a common progenitor cell for the three secretory lineages (24). However, the dependence of Paneth cell but not goblet or enteroendocrine cell differentiation on Wnt signaling suggests that differentiation of intestinal secretory lineages may involve a series of secretory progenitor cells that arise from cells expressing MATH1. A recent study describing increased numbers of enteroendocrine cells, reduced numbers of goblet cells, and absent Paneth cells in Gfi-1-null mice (25) is also consistent with additional secretory precursors segregating from MATH1-dependent cells.
The response to Wnt signaling and developmental requirement for these signals may be highly dependent on their timing for different cell lineages. Our observations provide direct evidence that enteroendocrine cells are specified independently of the Wnt pathway, whereas Paneth cells are not. In contrast to the Wnt, Notch signaling is one pathway that appears to be critical for enteroendocrine lineage specification through its effects on bHLH proteins (6, 7). In addition, the ability to activate Wnt target genes via the canonical Wnt pathway may be restricted to immature cells in the intestine and may be lost as cells differentiate. The features in the intestinal tumors reported here have not been previously described in mice with abnormal Wnt signaling and may provide further insight toward understanding the spectrum of neuroendocrine differentiation in human intestinal tumors.
Materials and Methods
Transgenic Mice.
To conditionally express a stabilized β-catenin allele in Ngn3- or NeuroD-expressing cells, Ngn3-Cre or NeuroD-Cre mice were crossed with the CatnbFlox(ex3)/+ line, which has loxP sites flanking the third exon. Genotyping for the Cre allele and CatnbFlox(ex3)/+ allele were performed as described previously. The offspring inherited a CatnbFlox(ex3)/+ allele, and either an Ngn3-Cre or NeuroD-Cre allele were analyzed for phenotypes.
Mice carrying an allele of β-catenin gene with loxP sites flanking gene sequence from exon 2 through exon 6 were obtained from The Jackson Laboratory (Catnbtm2kem /J) as homozygotes. Mice with one β-catenin gene null allele were generated by crossing Catnbtm2kem /J with EIIa-Cre mice, a general deletor strain from The Jackson Laboratory (B6.FVB-Tg (EIIa-Cre)C5379Lmgd/J). To conditionally delete β-catenin in Ngn3-expressing cells, a mating scheme was devised that only one conditional allele needs to undergo recombination to create tissue-specific null for β-catenin gene as described in ref. 19. The knockout animals were further crossed to homozygous R26R mice (B6.129S4-Gt 26 SortmSor) from The Jackson Laboratory (26) to generate mice with both β-catenin alleles deleted in Ngn3+ cells in a R26R background. Use of vertebrate animals was approved by the Institutional Animal Care and Use Committee at Tufts–New England Medical Center.
Histology and Immunohistochemistry.
Tissue samples were fixed in 4% paraformaldehyde and processed for paraffin sections. Slides were incubated with the following antibodies: mouse anti-β-catenin at 1:50 (Transduction Laboratories), rabbit anti-c-Myc at 1:100 (Santa Cruz Biotechnology), rabbit anti-chromogranin A at 1:1,000 (ImmunoStar), rabbit anti-serotonin at 1:10,000 (ImmunoStar), rabbit anti-secretin at 1:500 (W. Chey, University of Rochester, Rochester, NY), rabbit anti-cholecystokinin at 1:8,000 (Chemicon), rabbit anti-somatostatin at 1:3,000 (R. Lechan, Tufts University), rabbit anti-Ki67 at 1:1,000 (NeoMarkers), rabbit anti-PGP9.5 at 1:1,000 (UltraClone), and rabbit anti-lysozyme at 1:500 (Zymed). Immunoperoxidase labeling was performed with a VECTASTAIN ABC kit (Vector Laboratories) using 3,3′-diaminobenzidine precipitation for detection. X-Gal histochemistry was performed as described in ref. 12. Schiff's periodic acid staining kit (PolyScience) was used for PAS staining.
Analysis of DNA from Different Epithelial Cell Types for Recombination.
To identify enteroendocrine cells and enterocytes for isolation by laser microdissection, sections were either immunostained for chromogranin A or stained with hematoxylin/eosin, respectively. One thousand cells were isolated from tissue sections by laser microdissection with an Arcturus PixCell IIe system and digested overnight in 0.04% proteinase K/10 mM Tris·HCl (pH 8.0)/1 mM EDTA/1% Tween-20 at 37°C to isolate DNA. Cells were genotyped for β-catenin by PCR for 45–54 cycles using primers (5′-GCCTGGCCAGACTGCCTTTGT-3′ and 5′-GTCCACACAGCCCTGTCAAG-3′) that spanned the region of the gene containing exon 3.
RT-PCR Analysis.
Tissues were snap-frozen at the time of dissection in liquid nitrogen. RNA was extracted by using the Qiagen RNeasy Mini kit and reverse transcribed by using the Ambion RETROscript kit. RT-PCR analysis was carried out by using primers as follows: EphrinB3, 5′-AACAGCTTCCCCTGATTGTG-3′ and 5′-GACCCATAATGGAAGCCTCA-3′; c-myc, 5′-AGTGTTCTCTGCCTCTGCC-3′ and 5′-GCTCTGCTGTTGCTGGTGATA-3′; and CD44, 5′-CAGAAGAAAAAGCTGGTG-3′ and 5′-ACACCCCAATCTTCATGTCC-3′.
Acknowledgments
We thank Makoto M. Taketo (Kyoto University, Kyoto, Japan) for providing the CatnbFlox(ex3)/+ mice, the Tufts Transgenic Core Facility for assistance, and Greg Enders (University of Pennsylvania, Philadelphia, PA) for providing sections from APCmin mice. This work was supported in part by the Caring for Carcinoid Foundation; National Institutes of Health Grants DK43673, DK52870, and DK67166 (to A.B.L.); University of Parma Grant FIL04-06 (to G.R.); and the GRASP Digestive Disease Center through National Institutes of Health Grants P30-DK34928 and T32-DK007542.
Abbreviation
- NE
neuroendocrine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Gregorieff A, Clevers H. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 2.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 3.Pinto D, Gregorieff A, Begthel H, Clevers H. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M. Proc Natl Acad Sci USA. 1995;92:4482–4486. doi: 10.1073/pnas.92.10.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 7.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Proc Natl Acad Sci USA. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CS, Perreault N, Brestelli JE, Kaestner KH. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Diabetes. 2000;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- 11.Mutoh H, Naya FJ, Tsai MJ, Leiter AB. Genes Dev. 1998;12:820–830. doi: 10.1101/gad.12.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schonhoff SE, Giel-Moloney M, Leiter AB. Dev Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Bishop AE, Power RF, Polak JM. Pathol Res Pract. 1988;183:119–128. doi: 10.1016/s0344-0338(88)80040-2. [DOI] [PubMed] [Google Scholar]
- 14.Fujimori M, Ikeda S, Shimizu Y, Okajima M, Asahara T. Cancer Res. 2001;61:6656–6659. [PubMed] [Google Scholar]
- 15.Plockinger U, Rindi G, Arnold R, Eriksson B, Krenning EP, de Herder WW, Goede A, Caplin M, Oberg K, Reubi JC, et al. Neuroendocrinology. 2004;80:394–424. doi: 10.1159/000085237. [DOI] [PubMed] [Google Scholar]
- 16.Volante M, Rindi G, Papotti M. Virchows Arch. 2006;449:499–506. doi: 10.1007/s00428-006-0306-2. [DOI] [PubMed] [Google Scholar]
- 17.Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, et al. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prieve MG, Waterman ML. Mol Cell Biol. 1999;19:4503–4515. doi: 10.1128/mcb.19.6.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Development (Cambridge, UK) 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 20.Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, Laurent-Puig P, Kahn A, Robine S, Perret C, Romagnolo B. Development (Cambridge, UK) 2005;132:1443–1451. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 21.Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, et al. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 22.van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, et al. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 23.Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 25.Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriano P. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]