Abstract
Transformation of both prokaryotes and eukaryotes with single-stranded oligonucleotides can transfer sequence information from the oligonucleotide to the chromosome. We have studied this process using oligonucleotides that correct a −1 frameshift mutation in the LYS2 gene of Saccharomyces cerevisiae. We demonstrate that transformation by oligonucleotides occurs preferentially on the lagging strand of replication and is strongly inhibited by the mismatch-repair system. These results are consistent with a mechanism in which oligonucleotides anneal to single-stranded regions of DNA at a replication fork and serve as primers for DNA synthesis. Because the mispairs the primers create are efficiently removed by the mismatch-repair system, single-stranded oligonucleotides can be used to probe mismatch-repair function in a chromosomal context. Removal of mispairs created by annealing of the single-stranded oligonucleotides to the chromosomal DNA is as expected, with 7-nt loops being recognized solely by MutSβ and 1-nt loops being recognized by both MutSα and MutSβ. We also find evidence for Mlh1-independent repair of 7-nt, but not 1-nt, loops. Unexpectedly, we find a strand asymmetry of mismatch-repair function; transformation is blocked more efficiently by MutSα on the lagging strand of replication, whereas MutSβ does not show a significant strand bias. These results suggest an inherent strand-related difference in how the yeast MutSα and MutSβ complexes access and/or repair mismatches that arise in the context of DNA replication.
Keywords: Mlh1, Msh2, Msh3, Msh6, Saccharomyces cerevisiae
The transformation of yeast by single-stranded oligonucleotides (ssOligos) was first demonstrated 20 years ago by Sherman and colleagues (1). By using the CYC1 gene as a target of ssOligos of 50 nt, it was found that mismatches, in general, reduced ssOligo transformation (ssOT) efficiency, that 3′ deoxyoligonucleotides transformed somewhat less well than those with a 3′ hydroxyl and that ssOT was independent of tested recombination functions (2, 3). A strong strand bias also was observed, with ssOligos having the sequence of the coding strand of CYC1 (defined here as COD oligonucleotides) transforming 50–100 times better than oligonucleotides with the complementary sequence (noncoding, or NC, oligonucleotides). It was suggested that the differences between COD and NC ssOT frequencies were not related to transcriptional differences but could be due to preferential incorporation of oligonucleotides into either the leading or lagging strand of replication (3). More recently, the Kmiec (4) lab has studied the transformation of cyc1 point mutants using 70-nt ssOligos with three phosphorothioate bonds at the 3′ and 5′ termini. In this case, there appeared to be an opposite strand bias, with NC ssOligos transforming better than COD ssOligos. Furthermore, it was suggested that this transformation was enhanced by repair processes such as mismatch repair (MMR), with transformation apparently decreasing in MMR-defective cells (4).
In addition to the yeast studies, it has also been shown that ssOligos can transform Escherichia coli and correct the sequence of mutant genes. This process is RecA-independent and λ red-dependent, requiring only the β-protein (5). Recent work suggests that the β-protein is not required in the absence of ExoI and RecJ (6). A key discovery was the finding that MMR decreased transformation efficiency by as much as 100-fold, depending on the ssOligo sequence. Furthermore, in the absence of MMR, there was a strong bias for incorporation of the ssOligo annealing to the lagging strand of replication, and the effect of ssOligo sequence was eliminated (7, 8). The model of ssOT that has emerged from studies in E. coli is that the ssOligos pair with single-stranded regions of the chromosome, which are preferentially found on the lagging-strand template at the replication fork and that the mismatch-repair system strongly inhibits this process. It has also been demonstrated that ssOligos can transform mammalian cells (9–16). Although the mechanism of ssOT is controversial in mammalian cells, there are reports that the process is inhibited by the MMR system (13, 15, 16), and that the process is more efficient during S phase (9, 11, 12, 14).
DNA MMR in yeast removes mutational intermediates and blocks recombination between sequences with mismatches (17, 18). The recognition of mismatches is performed by two protein heterodimers containing proteins homologous to the MutS protein of E. coli: Msh2/Msh6 (MutSα) recognizes base/base mismatches and small insertion/deletion loops, whereas Msh2/Msh3 (MutSβ) primarily recognizes larger loops (19–21). MMR in addition requires interaction with one of several heterodimers of proteins homologous to E. coli MutL. The exact function of these heterodimers is not yet understood, but all appear to consist of Mlh1 usually paired with Pms1 but in rarer cases with Mlh2 or Mlh3 (22–24).
We report here on the transformation of the yeast chromosomal LYS2 locus by ssOligos in the presence/absence of defined MMR components. Because the direction of replication through the LYS2 locus has been established (25), one can assign an ssOligo as annealing to the leading- or lagging-strand template of DNA replication. We find that ssOT efficiency is reduced by the MMR system and depends on direction of replication through the LYS2 gene. The mechanism thus seems consistent with that observed in both E. coli and mammalian cells (5, 7, 8, 12–14). Importantly, we find a previously undetected difference in strand bias for the yeast MutS-like complexes, with MutSα being more effective for repairing mismatches on the lagging strand of replication and MutSβ having similar activities on both strands. These results establish ssOT in yeast as a powerful system for studying the repair of defined mismatches in a natural chromosomal setting.
Results
Transformation of Yeast by ssOligos Is Efficient.
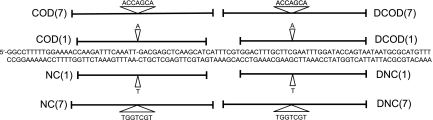
Reversion of the lys2ΔA746 −1 frameshift allele is ideal for ssOT because the corresponding region of Lys2 is insensitive to amino acid changes (26) and can thus be reverted by a compensatory net +1 frameshift anywhere within a theoretical reversion window of 150 bp. ssOligos used here contained either 1 nt or 7 nt of additional bases to correct the lys2ΔA746 −1 frameshift allele plus flanking sequences of 14–17 nt perfect identity. Even with ssOligos of 30 nt, transformation of WT cells was easily measurable, ranging from 1 × 10−4 to 1 × 10−3 per viable cell. For each oligonucleotide type, two different contexts were used, with ssOligos annealing to both the coding and noncoding strands of the LYS2 gene (Fig. 1). In its original chromosomal context, the LYS2 gene is replicated primarily from an upstream origin (25). Therefore the COD ssOligos, which have the same sequence as the coding strand of LYS2, would anneal to the leading strand of replication, and the NC ssOligos would anneal to the lagging strand of replication. To compare each specific ssOligo in both a leading and lagging strand context, the LYS2 gene was inverted relative to the upstream replication origin. To control for transformation efficiency between repetitions and between strains, a HIS3-containing CEN plasmid was included in all transformations, and the ratio of Lys+/His+ colonies was used as a measure of relative transformation efficiency.
Fig. 1.
Location of the ssOligos. Shown is a portion of the LYS2 sequence from strain SJR922 (26). The location of the −1 deletion is indicated by −, and the sequence of the coding strand is on top. COD and NC oligonucleotides are shown above and below the sequence, respectively. Nucleotides added by each ssOligo are indicated.
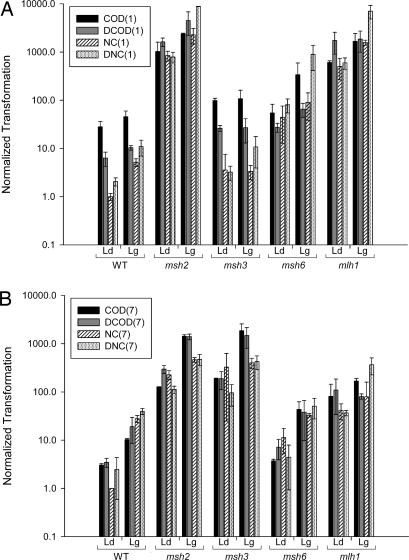
There is a wide range of transformation efficiencies among the different oligonucleotides in WT strains (Fig. 2), suggesting that the sequence of the oligonucleotide has a substantial effect on its transformation. For the +1 ssOligos, there is a weak tendency for a given ssOligo to transform best to the lagging strand; for the +7 ssOligos, transformation on the lagging is clearly more efficient.
Fig. 2.
ssOT is affected by MMR. The ratio of ssOT to control plasmid transformation was normalized to a value of 1.0 for transformation of the NC (1) or NC (7) ssOligo when annealing to the leading strand of replication (A and B, respectively). Error bars represent standard deviations from two or more experiments. For each genetic background, transformation to the leading strand (Ld) versus lagging strand (Lg) is indicated.
Mismatch Repair Inhibits ssOT.
ssOT in E. coli is markedly reduced by components of the MMR system (8), whereas similar transformation studies in yeast concluded that ssOT was enhanced by the MMR machinery (4, 27). To reexamine this issue in yeast, ssOligos were used to transform various MMR-defective mutants. It is immediately clear from Fig. 2 that ssOT for both +1 and +7 ssOligos is much more efficient in the absence of MMR (e.g., in msh2 strains). In addition, much of the effect of oligonucleotide sequence is eliminated in the absence of MMR. We conclude, as in E. coli, that ssOT in yeast is reduced by the MMR system.
Given what is known about MMR, ssOligos that introduce a 1-nt loop should be recognized by both MutSα and MutSβ, whereas ssOligos that introduce a 7-nt loop should be recognized only by MutSβ (17, 18). Transformation data for msh3 and msh6 strains (containing only MutSα and MutSβ, respectively) are shown in Fig. 2, and Table 1 presents a numerical comparison of ssOT efficiencies. Because of the effects of both ssOligo sequence and replication strand, it is important for comparisons to be done with each ssOligo individually and with transformation on the same strand of replication. For the +7 ssOligos, the average transformation increase in MMR-deficient strains is 80-fold (msh2/WT in Table 1). A similar increase is observed in strains lacking MutSβ (msh3/WT, msh2/msh3), whereas strains lacking MutSα are generally not significantly different from WT strains (msh6/WT). These results are consistent with the recognition of the resulting 7-nt loops by MutSβ but not MutSα. As expected from the dual recognition by MutSα and MutSβ, the situation is more complex with the +1 ssOligos. There is an average increase of 410-fold in transformation efficiency in completely MMR-deficient (msh2/WT) strains. In contrast to the results with the +7 ssOligos, neither the loss of MutSα nor MutSβ individually is sufficient to relieve all MMR suppression of transformation. MutSα is the dominant player in this case, because its loss results in an average 25-fold increase in ssOT (msh6/WT), whereas the loss of MutSβ does not yield a significant increase in ssOT (msh3/WT). The role of MutSβ in +1 ssOligo recognition, however, is indicated by the continued suppression of ssOT in the absence of MutSα, (msh6/WT and msh2/msh6 ratios).
Table 1.
Effect of MMR on ssOT
| ssOligo | msh2/WT | msh3/WT | msh6/WT | mlh1/WT | msh2/msh3 | msh2/msh6 | msh2/mlh1 |
|---|---|---|---|---|---|---|---|
| COD(1) leading | 37* | 3.5* | 1.9 | 22* | 10* | 19* | 1.7 |
| DCOD(1) leading | 260* | 4.1* | 4.3* | 270* | 62* | 59* | 0.9 |
| NC(1) leading | 840* | 3.6 | 44* | 500* | 240* | 19* | 1.7 |
| DNC(1) leading | 380* | 1.6 | 39* | 290* | 240* | 9.8* | 1.3 |
| COD(1) lagging | 52* | 2.4 | 7.5* | 37* | 22* | 7.0* | 1.4 |
| DCOD(1) lagging | 440* | 2.6* | 6.3* | 180* | 170* | 69* | 2.4 |
| NC(1) lagging | 440* | 0.6 | 18* | 300* | 680* | 25* | 1.5 |
| DNC(1) lagging | 810* | 1.0 | 82* | 650* | 820* | 9.9* | 1.3 |
| Average | 410 | 2.4 | 25 | 280 | 280 | 27 | 1.5 |
| COD(7) leading | 42* | 62* | 1.2* | 27* | 0.7* | 34* | 1.6 |
| DCOD(7) leading | 85* | 54* | 2.1 | 32* | 1.6 | 41* | 2.7* |
| NC(7) leading | 230* | 330* | 11* | 41* | 0.7 | 20* | 5.5* |
| DNC(7) leading | 46* | 39* | 1.8 | 15* | 1.2 | 25* | 3.1* |
| COD(7) lagging | 140* | 180* | 4.2* | 16* | 0.8 | 33* | 8.5* |
| DCOD(7) lagging | 73* | 77* | 2.0 | 4.2* | 0.9 | 37* | 17* |
| NC(7) lagging | 17* | 14* | 1.2 | 2.8 | 1.2 | 14* | 5.9* |
| DNC(7) lagging | 12* | 11* | 1.3 | 9.2* | 1.1 | 9.4* | 1.3 |
| Average | 80 | 96 | 3.1 | 18 | 1 | 27 | 5.7 |
For a given ssOligo, the normalized transformation values (Fig. 2) were used to compute the indicated ratios.
*A ratio in which the standard deviations of the denominator and numerator do not overlap; we consider such ratios to be different from 1.
In MMR, mismatches are recognized by proteins homologous to MutS, and then in conjunction with proteins homologous to MutL mismatched bases are excised and the newly replicated strand of DNA is resynthesized (18, 28). Whereas MutS and MutL proteins are equivalently necessary for replication-associated MMR, MutL proteins have less effect than MutS proteins in blocking recombination between mismatched sequences, indicating that the antimutation and antirecombination function of these proteins may be distinct (29–32). Because all MMR functions in yeast are thought to involve Mlh1 as one part of a heterodimer with other MutL proteins, we deleted MLH1 and measured ssOT (Fig. 2 and Table 1). With the +1 oligonucleotides, there was no case in which the transformation in mlh1 cells was significantly different from that in msh2 cells, suggesting that the suppression of ssOT by Mlh1 is due to mismatch repair activity and not an antirecombination activity. ssOT of the +7 ssOligos was different, however, with MSH2 deletion increasing ssOT an average of 5-fold more than MLH1 deletion (Table 1, msh2/mlh1). The unexpected results with the +7 ssOligos may be related to the recent finding of an MLH1-independent meiotic activity involved in the repair of 4-nt loop mismatches (33).
Transformation Is Affected by the Direction of DNA Replication.
Inversion of the LYS2 locus reverses the leading- and lagging-strand templates but does not alter the transcriptional status of the chromosomal sequences. Although the inversion of LYS2 resulted in the disruption of the adjacent RAD16 gene, disruption of RAD16 in the original orientation had essentially no effect on ssOT efficiencies (data not shown). In the WT orientation, the coding and noncoding strands of LYS2 serve as the templates for lagging- and leading-strand synthesis, respectively; in the inverted orientation, the coding and noncoding strands serve as the templates for leading- and lagging-strand synthesis, respectively.
The ssOT results from Fig. 2 are summarized in Table 2, where the ratio of the lagging- to leading-strand transformation efficiency is given for each individual ssOligo in different genetic backgrounds. These results clearly show that in the complete absence of MMR (msh2 cells), ssOligos preferentially transform on the lagging strand of replication. There is one condition, however, in which the lagging strand is not transformed at a significantly higher level than the leading strand: in ssOT with the +1 ssOligos when cells contain only MutSα (msh3 cells). The elimination of the lagging strand bias in cells with only MutSα active indicates that MutSα has a greater activity on the lagging strand than the leading strand, consistent with previous observations (34). For ssOT with the +7 ssOligos, where MutSβ has the only significant activity, the lagging/leading strand transformation bias clearly is not reduced but, instead, is modestly increased in the presence of MutSβ; this is evident in both WT and msh6 cells. These results strongly indicate that MutSβ does not have a lagging-strand bias as does MutSα and suggest that MutSβ may instead have a leading-strand bias.
Table 2.
Effect of transformation of lagging versus leading strand
| ssOligo | WT | msh2 | msh3 | msh6 | mlh1 |
|---|---|---|---|---|---|
| COD(1) lag/lead | 1.6 | 2.3* | 1.1 | 6.2* | 2.7* |
| DCOD(1) lag/lead | 1.6* | 2.8* | 1.0 | 2.4* | 1.1 |
| NC(1) lag/lead | 5.2* | 2.7* | 0.9 | 2.0 | 3.1* |
| DNC(1) lag/lead | 5.3* | 11* | 3.3 | 11* | 12* |
| Average | 3.4 | 4.7 | 1.6 | 5.4 | 4.7 |
| COD(7) lag/lead | 3.4* | 11* | 9.8* | 12* | 2.1* |
| DCOD(7) lag/lead | 5.5* | 4.7* | 7.8* | 5.3 | 0.7 |
| NC(7) lag/lead | 28* | 2.0* | 1.2 | 2.9* | 1.9 |
| DNC(7) lag/lead | 16* | 4.2* | 4.4* | 11* | 10* |
| Average | 13 | 5.6 | 5.8 | 7.8 | 3.7 |
Effect of Transcriptional Strand on Transformation.
The results of comparison of ssOligos annealing to the transcribed versus nontranscribed strands (COD and NC, respectively) is shown in Table 3 for both +1 and +7 ssOligos. For the +7 ssOligos, there do not seem to be any clear trends with respect to transcriptional strand. For the +1 ssOligos, transformation to either strand is approximately equal when MutSα is absent. In the presence of MutSα (in WT or msh3 strains), transformation with a COD or DCOD ssOligo (annealing to the transcribed strand) is significantly higher compared with the NC or DNC ssOligos (annealing to the nontranscribed strand). This would suggest that MutSα is more active on the nontranscribed strand than on the transcribed strand. This asymmetry might be related to previous work that has shown subtle effects of transcription on MMR (35).
Table 3.
Effect of transformation on coding versus noncoding strand
| ssOligo | WT | msh2 | msh3 | msh6 | mlh1 |
|---|---|---|---|---|---|
| COD(1)/NC(1) lead | 28* | 1.2 | 28* | 1.2 | 1.2 |
| DCOD(1)/DNC(1) lead | 3.1* | 2.1* | 8.1* | 0.3* | 2.9* |
| COD(1)/NC(1) lag | 8.8* | 1.0 | 32* | 3.8 | 1.1 |
| DCOD(1)/DNC(1) lag | 1.0 | 0.5* | 2.5 | 0.1* | 0.3* |
| Average | 10 | 1.2 | 18 | 1.3 | 1.4 |
| COD(7)/NC(7) lead | 3.0* | 0.6 | 0.6 | 0.3* | 2.0 |
| DCOD(7)/DNC(7) lead | 1.4 | 2.6 | 2.0 | 1.6 | 3.0 |
| COD(7)/NC(7) lag | 0.4* | 3.1* | 4.6* | 1.3 | 2.1 |
| DCOD(7)/DNC(7) lag | 0.5* | 2.9* | 3.5* | 0.7 | 0.2 |
| Average | 1.3 | 2.3 | 2.7 | 1.0 | 1.8 |
Effect of Recombination Genes on Transformation.
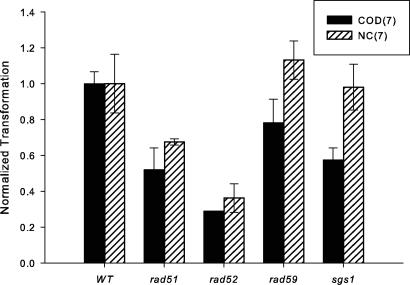
There have been varying reports in the literature about the requirement of annealing and recombination functions for ssOT (2, 4). We therefore examined the effect of deletion of the RAD51, RAD52, and RAD59 genes on ssOT in our system. In yeast, Rad51 and Rad59 are important for strand-invasion and strand-annealing types of recombination, respectively, whereas Rad52 is required for all types of recombination (36). There were 2- to 4-fold decreases in transformation efficiency in either rad51 or rad52 strains, with a greater decrease in the rad52 strain (Fig. 3). Deletion of RAD59 had little, if any effect. After recognition of mismatches in the annealed oligonucleotide, if removal requires a helicase, then removal of the necessary helicase might increase transformation by blocking oligonucleotide removal. Therefore, we tested the effect of the helicase Sgs1, known to be involved in the antirecombination activity of MMR proteins (30, 37). Transformation in sgs1 strains was unchanged on the lagging strand and decreased slightly on the leading strand (Fig. 3). In agreement with the work from Sherman's lab (2), we have not detected a strong dependence of ssOT on recombination genes.
Fig. 3.
The effect of recombination genes on ssOT. The indicated ssOligos were transformed into derivatives of SJR922, and the ratio of ssOT to control plasmid transformation were normalized to the value of the ssOligo in the WT strain. Error bars are as in Fig. 2.
Discussion
We have used ssOligos of 30–40 nt in length annealing to either DNA strand in two different locations in the LYS2 gene to correct a −1 frameshift by the addition of 1 or 7 nt. In addition, we have performed these experiments with the LYS2 gene inverted with respect to a nearby origin of replication. Our results show that MMR strongly blocks ssOT in a sequence-specific manner, and have allowed us to study certain aspects of MMR in vivo in unprecedented detail. We find that, on average, MMR recognized +1 loops more efficiently than +7 loops. As expected, the complexes formed by the +7 ssOligos are recognized only by MutSβ and not by MutSα. In contrast to the situation with the +7 ssOligos, complexes formed by the +1 ssOligos are recognized by both MutSα and MutSβ. The weaker phenotype due to MutSβ deficiency could be due to different amounts of MutSα and MutSβ in the cells or differing affinities for +1 loops. For example, there could be sufficient MutSα in cells to recognize most complexes of the ssOligo on the DNA but a lower amount of MutSβ that would not be present at all replication complexes. A lower amount of MutSβ would also be consistent with the more efficient overall recognition of +1 loops compared with +7 loops, as would a weaker affinity of MutSβ for +1 loops. It is not clear why the effect of MMR in our system differs from that reported previously in yeast (4, 27). We note that the earlier experiments used longer ssOligos of 74 nt, with three phosphorothioate linkages on both ends, and it may be that their mechanism of incorporation is different. The results reported here are, however, consistent with experiments in both E. coli (7, 8) and mammalian cells (13, 15, 16) as well as those from Sherman's lab in yeast (1–3).
There have been no previous reports of the effect of homologues of MutL on ssOT. For transformation with the +1 ssOligos, we find the deletion of MLH1 is indistinguishable from deletion of MSH2. This finding is consistent with the requirement of both MutS and MutL complexes in MMR. Thus, it was particularly surprising to find a very different situation for transformation of +7 ssOligos, in which ssOT is 5-fold more efficient in msh2 than in mlh1 cells. Either there is a different mechanism of rejection of ssOT with +7 ssOligos, or there is an MLH1-independent activity that removes only larger loops (33). With regard to the latter possibility, it has recently been found that there is some meiotic repair of 4-nt loops that requires Mlh3 but not Mlh1 (33). Although it has been known for some time that repair of large loops (larger than those used here) is independent of MMR (38), indication of any mitotic MMR function that depends on Msh2, but not Mlh1, has not been previously shown.
It has been reported that MMR is 4- to 5-fold more active on the lagging strand of replication, and it was suggested that this difference could be due to the greater numbers of nicks or higher density of PCNA on the lagging strand (34). Those experiments measured repair of single base mismatches and so specifically examined MutSα activity. Our results are consistent with those results, because ssOT was inhibited 3-fold more by MutSα on the lagging strand than on the leading strand. The expectation was that MutSβ would exert a similar bias on ssOT, but that is clearly not the case. Our results indicate that MutSβ activity is distributed approximately equally on both the leading and lagging strands of replication or may even have a slight leading-strand bias. MutSβ activity is thus distributed differently from MutSα activity. The greater activity of MutSα on the lagging strand most likely reflects an asymmetric distribution of protein, although the mechanism that might establish strand asymmetry for MutSα and MutSβ is not clear. Both complexes interact with PCNA, however, through either Msh3 or Msh6 but not Msh2 (39). Furthermore, there appear to be differences in the way that PCNA interacts with Msh3 compared with Msh6 (40), and the differences in these interactions could be a possible mechanism for establishing the observed strand asymmetry. In addition, it is generally accepted that leading and lagging strands are replicated by different DNA polymerases, providing another potential mechanism for establishing asymmetry for the yeast MutS complexes.
Although our results establish a strand asymmetry of MutSα and MutSβ during the repair of mismatches introduced by ssOT, the biological relevance of this asymmetry is difficult to assess. There are several observations in the literature, however, that have never been satisfactorily explained, and we suggest that these could reflect a strand asymmetry of MMR. Assays for dinucleotide repeat slippage, for example, found that 87% of events were due to a gain of repeat units in msh6 strains, whereas 85% of events were due to a loss in msh3 strains (41, 42). Similarly, an examination of loss or gain of bases in mononucleotide repeats found a complex pattern that depended not only on the genetic background but also on whether there were Cs or Gs on the coding strand (43). In msh3 cells, 85% of the slippage events were losses when Cs were in the coding strand, whereas 98% of slippage events were gains when Gs were in the coding strand. This bias was reversed in msh6 cells. None of the mono- or dinucleotide results are easily explained if MutSα and MutSβ have the same distribution of activity on the two strands of replication.
The results reported here demonstrate that transformation of yeast by ssOligos is sensitive to MMR as has been observed in both bacteria and mammalian cells. Mechanistically, the results are consistent with a model in which the observed bias toward the lagging strand of replication results from the greater single-stranded character of that strand, providing more opportunity for the annealing of ssOligos. It is important to emphasize that the results obtained from ssOT are not only consistent with other studies of in vivo MMR but have provided insights into this process. Specifically, the use of ssOligos with differing sensitivities to MutSα and MutSβ has revealed striking and unexpected strand differences in the activity of the two MMR complexes as well as unexpected differences in response to loss of MLH1. With a better understanding of the mechanism in place, this method should prove extremely valuable in studying the effects of defined base mismatches and damage on repair and replication in a chromosomal context (44–47).
Materials and Methods
Strain Construction.
All strains were derivatives of SJR922 [MATα ade2–101oc his3Δ200 ura3ΔNco lys2ΔA746 (26)] and are listed in supporting information (SI) Table 4. A 4.9-kb XbaI/HindIII fragment containing the LYS2 gene and a part of the downstream RAD16 gene was inverted by transforming a lys2-parent strain with this sequence joined to switched flanking sequences. The lys2ΔA746 allele was then introduced by two-step allele replacement (26). RAD52 was deleted by transformation with BamHI/EcoRI-digested pSR136 (48). All other genes were deleted by transformation with a PCR product amplified from the appropriate gene-deletion strain and conferring G418R (49).
Transformation with ssOligos.
Yeast transformation was performed by electroporation following the general procedure of Otsuka et al. (46). Briefly, an overnight culture of yeast cells (25 ml) was inoculated into 900 ml of YPDA medium (yeast–peptone–dextrose containing 0.0075% l-adenine hemisulfate) and cultured until the OD600 reached 1.3–1.5. Cells were collected by centrifugation at 4°C, washed twice with sterile cold double-distilled H2O and once with cold 1 M sorbitol, and resuspended in 900 μl of sterile 1 M sorbitol. In a typical experiment, 300 pmol of ssOligo and 10 ng of the control plasmid pRS313 (50) were added to 300 μl of the competent cell suspension; this was in the linear range of transformation efficiencies. The mixture was kept on ice for 5 min and transferred to an electroporation cuvette (2-mm gap, 400-μl capacity). Electroporation was performed by applying a 1.5 kV, 186 Ω pulse (Electro Cell Manipulator Electroporation System; BTX, San Diego, CA). The cells were then diluted immediately with 900 μl of YPDA medium and allowed to recover with gentle agitation at 30°C for 15 min before being spread on synthetic dextrose (SD) medium lacking the appropriate amino acid (51) to select transformants. Colonies were counted after 5- to 7-days incubation at 30°C.
Oligonucleotides were obtained from Operon (Huntsville, AL) and purified by electrophoresis on a 15% polyacrylamide gel (52). All transformations were done with the same mixture of oligonucleotide and plasmid. Transformation efficiencies were normalized across strains by calculating the ratio of oligonucleotide to plasmid transformants. The least efficient transformations were at least 10-fold higher than levels of background reversion, and sequencing of selected transformants demonstrated that all acquired sequences were from the ssOligos.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants CA54050 (to G.F.C.), GM038464 (to S.J.-R.), and CA90860 (to Y.W.K.), National Institute on Environmental Health Sciences Grant P01 ES011163 (to Y.W.K.), and the University Research Committee of Emory University (G.F.C.).
Abbreviations
- ssOligos
single-stranded oligonucleotides
- ssOT
single-stranded oligonucleotide transformation
- COD
having the sequence of the coding strand
- NC
having the sequence of the noncoding strand
- MMR
mismatch repair.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704695104/DC1.
References
- 1.Moerschell RP, Tsunasawa S, Sherman F. Proc Natl Acad Sci USA. 1988;85:524–528. doi: 10.1073/pnas.85.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto T, Moerschell RP, Wakem LP, Ferguson D, Sherman F. Yeast. 1992;8:935–948. doi: 10.1002/yea.320081104. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Moerschell RP, Wakem LP, Komar-Panicucci S, Sherman F. Genetics. 1992;131:811–819. doi: 10.1093/genetics/131.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brachman EE, Kmiec EB. Genetics. 2003;163:527–538. doi: 10.1093/genetics/163.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis HM, Yu D, DiTizio T, Court DL. Proc Natl Acad Sci USA. 2001;98:6742–6746. doi: 10.1073/pnas.121164898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutra BE, Sutera VA, Jr, Lovett ST. Proc Natl Acad Sci USA. 2007;104:216–221. doi: 10.1073/pnas.0608293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costantino N, Court DL. Proc Natl Acad Sci USA. 2003;100:15748–15753. doi: 10.1073/pnas.2434959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li XT, Costantino N, Lu LY, Liu DP, Watt RM, Cheah KSE, Court DL, Huang JD. Nucleic Acids Res. 2003;31:6674–6687. doi: 10.1093/nar/gkg844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radecke S, Radecke F, Peter I, Schwarz K. J Gene Med. 2006;8:217–228. doi: 10.1002/jgm.828. [DOI] [PubMed] [Google Scholar]
- 10.Bertoni C, Morris GE, Rando TA. Hum Mol Genet. 2005;14:221–233. doi: 10.1093/hmg/ddi020. [DOI] [PubMed] [Google Scholar]
- 11.Olsen PA, Randol M, Krauss S. Gene Ther. 2005;12:546–551. doi: 10.1038/sj.gt.3302454. [DOI] [PubMed] [Google Scholar]
- 12.Wu XS, Xin L, Yin WX, Shang XY, Lu L, Watt RM, Cheah KSE, Huang JD, Liu DP, Liang CC. Proc Natl Acad Sci USA. 2005;102:2508–2513. doi: 10.1073/pnas.0406991102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekker M, Brouwers C, te Riele H. Nucleic Acids Res. 2003;31:e27. doi: 10.1093/nar/gng027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Muyrers JP, Rientjes J, Stewart AF. BMC Mol Biol. 2003;4:1. doi: 10.1186/1471-2199-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aarts M, Dekker M, de Vries S, van der Wal A, te Riele H. Nucleic Acids Res. 2006;34:e147. doi: 10.1093/nar/gkl896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekker M, Brouwers C, Aarts M, van der Torre J, de Vries S, van de Vrugt H, te Riele H. Gene Ther. 2006;13:686–694. doi: 10.1038/sj.gt.3302689. [DOI] [PubMed] [Google Scholar]
- 17.Harfe BD, Jinks-Robertson S. Annu Rev Genet. 2000;34:359–399. doi: 10.1146/annurev.genet.34.1.359. [DOI] [PubMed] [Google Scholar]
- 18.Crouse GF. In: DNA Damage and Repair, Volume 1: DNA Repair in Prokaryotes and Lower Eukaryotes. Nickoloff JA, Hoekstra MF, editors. Totowa, NJ: Humana; 1998. pp. 411–448. [Google Scholar]
- 19.Kolodner RD, Marsischky GT. Curr Opin Genet Dev. 1999;9:89–96. doi: 10.1016/s0959-437x(99)80013-6. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RE, Kovvali GK, Prakash L, Prakash S. J Biol Chem. 1996;271:7285–7288. doi: 10.1074/jbc.271.13.7285. [DOI] [PubMed] [Google Scholar]
- 21.Marsischky GT, Filosi N, Kane MF, Kolodner R. Genes Dev. 1996;10:407–420. doi: 10.1101/gad.10.4.407. [DOI] [PubMed] [Google Scholar]
- 22.Harfe BD, Minesinger BK, Jinks-Robertson S. Curr Biol. 2000;10:145–148. doi: 10.1016/s0960-9822(00)00314-6. [DOI] [PubMed] [Google Scholar]
- 23.Wang TF, Kleckner N, Hunter N. Proc Natl Acad Sci USA. 1999;96:13914–13919. doi: 10.1073/pnas.96.24.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flores-Rozas H, Kolodner RD. Proc Natl Acad Sci USA. 1998;95:12404–12409. doi: 10.1073/pnas.95.21.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freudenreich CH, Stavenhagen JB, Zakian VA. Mol Cell Biol. 1997;17:2090–2098. doi: 10.1128/mcb.17.4.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harfe BD, Jinks-Robertson S. Mol Cell Biol. 1999;19:4766–4773. doi: 10.1128/mcb.19.7.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maguire KK, Kmiec EB. Gene. 2007;386:107–114. doi: 10.1016/j.gene.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunkel TA, Erie DA. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 29.Goldfarb T, Alani E. Genetics. 2005;169:563–574. doi: 10.1534/genetics.104.035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugawara N, Goldfarb T, Studamire B, Alani E, Haber JE. Proc Natl Acad Sci USA. 2004;101:9315–9320. doi: 10.1073/pnas.0305749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson A, Hendrix M, Jinks-Robertson S, Crouse GF. Genetics. 2000;154:133–146. doi: 10.1093/genetics/154.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spell RM, Jinks-Robertson S. Genetics. 2003;165:1733–1744. doi: 10.1093/genetics/165.4.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone JE, Petes TD. Genetics. 2006;173:1223–1239. doi: 10.1534/genetics.106.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlov YI, Mian IM, Kunkel TA. Curr Biol. 2003;13:744–748. doi: 10.1016/s0960-9822(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 35.Wierdl M, Greene CN, Datta A, Jinks-Robertson S, Petes TD. Genetics. 1996;143:713–721. doi: 10.1093/genetics/143.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krogh BO, Symington LS. Annu Rev Genet. 2004;38:233–271. doi: 10.1146/annurev.genet.38.072902.091500. [DOI] [PubMed] [Google Scholar]
- 37.Spell RM, Jinks-Robertson S. Genetics. 2004;168:1855–1865. doi: 10.1534/genetics.104.032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corrette-Bennett SE, Mohlman NL, Rosado Z, Miret JJ, Hess PM, Parker BO, Lahue RS. Nucleic Acids Res. 2001;29:4134–4143. doi: 10.1093/nar/29.20.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark AB, Valle F, Drotschmann K, Gary RK, Kunkel TA. J Biol Chem. 2000;275:36498–36501. doi: 10.1074/jbc.C000513200. [DOI] [PubMed] [Google Scholar]
- 40.Lau PJ, Flores-Rozas H, Kolodner RD. Mol Cell Biol. 2002;22:6669–6680. doi: 10.1128/MCB.22.19.6669-6680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sia EA, Kokoska RJ, Dominska M, Greenwell P, Petes TD. Mol Cell Biol. 1997;17:2851–2858. doi: 10.1128/mcb.17.5.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strand M, Earley MC, Crouse GF, Petes TD. Proc Natl Acad Sci USA. 1995;92:10418–10421. doi: 10.1073/pnas.92.22.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gragg H, Harfe BD, Jinks-Robertson S. Mol Cell Biol. 2002;22:8756–8762. doi: 10.1128/MCB.22.24.8756-8762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kow YW, Bao G, Minesinger B, Jinks-Robertson S, Siede W, Jiang YL, Greenberg MM. Nucleic Acids Res. 2005;33:6196–6202. doi: 10.1093/nar/gki926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otsuka C, Sanadai S, Hata Y, Okuto H, Noskov VN, Loakes D, Negishi K. Nucleic Acids Res. 2002;30:5129–5135. doi: 10.1093/nar/gkf666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otsuka C, Kobayashi K, Kawaguchi N, Kunitomi N, Moriyama K, Hata Y, Iwai S, Loakes D, Noskov VN, Pavlov Y, et al. Mutat Res. 2002;502:53–60. doi: 10.1016/s0027-5107(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 47.Noskov V, Negishi K, Ono A, Matsuda A, Ono B, Hayatsu H. Mutat Res. 1994;308:43–51. doi: 10.1016/0027-5107(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 48.Freedman JA, Jinks-Robertson S. Genetics. 2002;162:15–27. doi: 10.1093/genetics/162.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, et al. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 50.Sikorski RS, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherman F. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 52.Yao M, Kow YW. J Biol Chem. 1997;272:30774–30779. doi: 10.1074/jbc.272.49.30774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.