Abstract
Neurons are flexible electrophysiological entities in which the distribution and properties of ionic channels control their behaviors. Through simultaneous somatic and axonal whole-cell recording of layer 5 pyramidal cells, we demonstrate a remarkable differential expression of slowly inactivating K+ currents. Depolarizing the axon, but not the soma, rapidly activated a low-threshold, slowly inactivating, outward current that was potently blocked by low doses of 4-aminopyridine, α-dendrotoxin, and rTityustoxin-Kα. Block of this slowly inactivating current caused a large increase in spike duration in the axon but only a small increase in the soma and could result in distal axons generating repetitive discharge in response to local current injection. Importantly, this current was also responsible for slow changes in the axonal spike duration that are observed after somatic membrane potential change. These data indicate that low-threshold, slowly inactivating K+ currents, containing Kv1.2 α subunits, play a key role in the flexible properties of intracortical axons and may contribute significantly to intracortical processing.
Keywords: axon, cortex, plasticity, synaptic transmission
The precise distribution and properties of ionic channels in cortical neurons strongly influence both the cell's intrinsic electrophysiological properties and its operation within cortical networks. Although the properties of cortical neuronal cell bodies and dendrites have been extensively studied, the study of neocortical axons has been largely confined to recordings from axonal segments near the cell body (e.g., see refs. 1 and 2).
Far from being simple static structures that merely communicate spikes, the dynamical properties of intracortical axons may contribute critically to the operation of local cortical networks (reviewed in ref. 3). Recently, it was shown that the amplitude of excitatory postsynaptic potentials evoked between excitatory neurons depends on the membrane potential of the presynaptic cell (4, 5). Modest somatic depolarization of presynaptic neocortical pyramidal cells increased the average excitatory postsynaptic potential (EPSP) amplitude evoked in nearby pyramidal cells. Interestingly, simultaneous axonal and somatic patch clamp recordings revealed that these somatic depolarizations increased axonal spike duration over a time course that was similar to the slow component of synaptic facilitation. These results suggest that information transmission within local cortical networks may operate in a mixed “analog” and “digital” mode (4, 5) and that axonal spike duration may be critically involved in this process, at least for some intracortical synaptic interactions.
What controls intracortical axonal spike duration? The answer to this question is largely unknown. Immunocytochemical studies indicate that pyramidal cell axons contain a high density of transient and persistent Na+ currents, particularly in the axon initial segment and nodes of Ranvier (6–8) and at least Kv1.2 α-subunit containing K+ channels (6). In other preparations, Kv1.1, 1.2, and 1.6 α-subunits contribute to the formation of low-threshold K+ channels that are important in determining axonal excitability and axonal spike duration (9–12). In addition, cortical synaptic transmission is strongly sensitive to low doses of 4-aminopyridine and the Kv1.1, 1.2, and 1.6 channel blocker α-dendrotoxin (13–15), indicating that K+ channels containing these subunits may be particularly important in presynaptic axons and terminals.
Here, we demonstrate that a low-threshold, slowly inactivating K+ current, consisting, at least in part, of Kv1.2 α subunits, plays a dominant role in controlling axonal excitability and spike duration in neocortical layer 5 pyramidal cell axons. The time and voltage dependence of this current may allow for dynamical changes in intracortical synaptic transmission that are important for cortical operation and information transfer (5).
Results
Simultaneous whole-cell recordings were obtained from both the soma and the main axon of ferret and rat prefrontal cortical layer 5 pyramidal cells (n = 53; Figs. 1 and 2) (1, 5). The axonal recordings were obtained at a distance of 45–310 μm from the cell body through patching the terminal bleb of the main axon at the surface of the slice (see Methods).
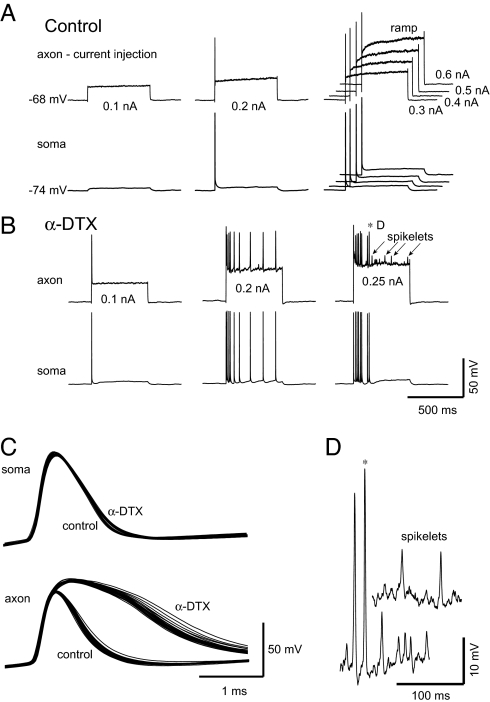
Fig. 1.
An α-dendrotoxin-sensitive current controls cortical axonal excitability. (A) Simultaneous somatic and axonal recording. The intraaxonal injection of current pulses results in only single spikes in both the axon and the soma, followed by a slow ramping depolarization of the axon. (B) After bath application of α-dendrotoxin (100 nM; α-DTX) axonal spike threshold is lower, trains of spikes are initiated with moderate amplitude current pulses, the slow depolarizing ramp of the axonal membrane potential in response to depolarization was blocked, and larger intraaxonal current pulses initiated “spikelets” that were not evident in the somatic recording. (C) The application of α-DTX strongly increased axonal spike duration but not in the soma (first spike to each pulse shown). (D) Expansion of two normal spikes and several “spikelets” (in α-DTX). Axon recorded at 110 μm (P20 rat prefrontal cortex).
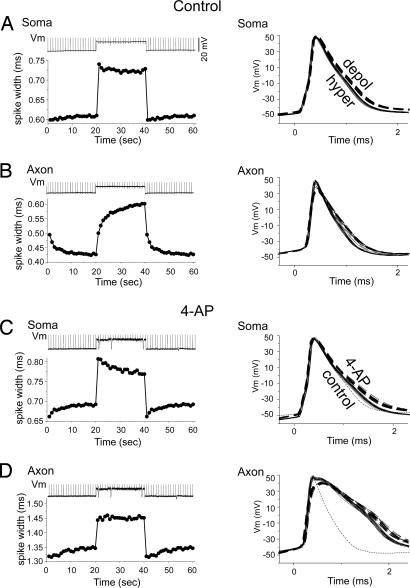
Fig. 2.
A 4-AP sensitive current controls slow changes in axonal spike duration. (A) Somatic depolarization rapidly, but only mildly, increases spike duration in the soma (n = 9; single cell traces are shown on right). (B) The somatic depolarization (from an average of −65 to −52 mV) also rapidly depolarizes the axon (top trace; example from one trial in one cell) and slowly increases axonal spike duration over 10s of seconds. (C) Bath application of 4-AP (40 μM) results in a small increase in somatic spike duration. Dashed trace (control) is a spike obtained at hyperpolarized levels before application of 4-AP. (D) The 4-AP increases axonal spike duration and blocks the time-dependent changes with membrane potential. The data presented on the left in A–D are the average of nine cells from ferret PFC. The raw data presented on the right are from one paired somatic-axonal recording (100 μm).
The intrasomatic injection of constant current initiated a train of spikes in regular spiking cortical pyramidal cells, with each spike propagating down the main axon (1). The injection of constant current pulses into distal (> 100 μm) axons in contrast, initiates single spikes only, followed by their back propagation into the soma (Fig. 1A). After this single spike, the axonal membrane potential exhibited a slow depolarizing ramp (Fig. 1A).
In other axonal preparations, the limitation of axonal responses to prolonged depolarization to a single spike results, in part, from the activation of low-threshold and α-dendrotoxin (α-DTX), 4-AP-sensitive K+ currents (reviewed in ref. 10). To test whether similar K+ currents may contribute to the control of axonal excitability in the neocortex, we examined the effects of low doses of α-DTX (100 nM; n = 15) and 4-AP (40 μM; n = 16). The bath application of α-DTX to rat layer 5 pyramidal cells reduced axonal spike threshold and converted the response from a single spike to a train of spikes (Fig. 1B). Examination of the dV/dt versus membrane potential phase plot of the somatic spikes revealed two components, indicating that they were initiated by back-propagating axonal spikes (16). The bath application of α-DTX blocked the slow depolarizing ramp that normally occurs with prolonged axonal depolarization (Fig. 1 A and B). After the application of α-DTX, the intraaxonal injection of larger depolarizing current pulses activated “spikelets,” which did not invade the soma (Fig. 1 B and D). We assume that these spikelets are either spikes that were generated in distal axonal locations or the failed local generation of spikes at or near the recording site (Fig. 1 B and D). Finally, the application of α-DTX dramatically increased the duration of axonal spikes (from 1.24 ± 0.23 msec control to 3.3 ± 1.3 msec, α-DTX, n = 5; P < 0.01) but only slightly broadened somatic spikes (Fig. 1C; 1.1 ± 0.1 msec control, 1.2 ± 0.1 msec, α-DTX; n = 5; P < 0.01). The prolongation of axonal spike duration occurred through a selective reduction of spike repolarization rate (Fig. 1C).
Slow Changes in Axonal Spike Duration Blocked by 4-Aminopyridine.
The duration and, to some extent, amplitude, of axonal spikes is strongly sensitive to somatic (and axonal) membrane potential (5). Here, we examined the possibility that the sensitivity of axonal spike duration may result from the properties of a 4-AP- and α-DTX-sensitive current. Spikes were initiated in the axon initial segment (1, 2, 17) through the intrasomatic injection of short duration (1–2 msec) depolarizing current pulses. Depolarization of the soma from resting membrane potentials (typically −62 to −72 mV) to near firing threshold (typically −49 to −55 mV) rapidly (within 50 msec) increases somatic spike duration (from an average of 0.61 ± 0.04 msec to 0.72 ± 0.05 msec; n = 9; P < 0.01; measured at half-height). Somatic spike duration often slowly decreased slightly after an initial peak with the onset of tonic depolarization (Fig. 2A). Removal of the somatic depolarizing constant current injection rapidly decreased somatic spike duration, which then slowly increased slightly over several seconds (Fig. 2A).
Axonal spikes behaved markedly different from those in the soma, even though they were recorded simultaneously. Although axonal membrane potential was depolarized rapidly by depolarization of the soma, the duration of axonal spikes was increased only over several seconds (Fig. 2B). This increase in duration could be well fit (r2 = 0.98) with a single exponential function with a time constant (τ) of 4.9 s or by a two-exponential function (r2 = 0.99) with τs of 0.58 s (38% contribution) and 7.58 s (62%) (n = 9). Upon removal of the somatic depolarization, the membrane potential of the axon returned to rest rapidly. However, axonal spike duration decreased slowly, with a time course that could be well fit (r2 = 0.97) with a τ of 2.54 seconds or with a two-exponential function (r2 = 0.99) with τs of 0.99 s (60%) and 5.28 s (40%) (n = 9). The membrane potential of the axon exhibited only small (< 1 mV) changes over the course of the depolarization and hyperpolarization, indicating that the changes in spike duration were not a consequence of changes in local membrane potential.
Studies have shown that low doses of 4-AP block a K+ current (D-current) that activates rapidly, inactivates slowly, and may contribute to spike repolarization (18–21). We tested the possibility that a K+ current that is highly sensitive to block by 4-AP may contribute to spike repolarization in the soma and axon of ferret layer 5 pyramidal cells. Bath application of 4-AP (40–50 μM) only slightly broadened somatic spikes at resting membrane potential (from 0.61 ± 0.04 to 0.685 ± 0.08 msec; n = 9; P < 0.01) as well as exaggerated the slow changes in somatic spike duration induced by tonic depolarization and hyperpolarization (Fig. 2C).
In contrast, 4-AP markedly increased the duration of the same spikes in the axon (Fig. 2D); increasing their duration to 1.39 ± 0.06 from 0.51 ± 0.07 msec (n = 5; P < 0.01). In the presence of 4-AP, depolarization of the soma had only a small, and rapid, effect on axonal spike duration. Similar results were obtained with bath application of α-DTX (100 nM; n = 4; not shown). Finally, bath application of low doses of 4-AP lowered spike threshold by −2.1 ± 0.2 mV in the axon and −3.0 ± 0.8 mV in the soma.
Cortical Axons Contain a Slowly Inactivating K+ Current.
Our results suggest that intracortical axons may contain slowly inactivating, 4-AP- and α-DTX-sensitive K+ currents that are critical to the repolarization of axonal spikes. To examine this possibility, we applied a series of voltage clamp pulses before and after the bath application of 4-AP and α-DTX (Fig. 3; in TTX, 1 μM). Axonal voltage steps (holding potential of −100 to −80 mV) activated a slowly inactivating outward current, that was blocked by 4-AP (Fig. 3 A–C; n = 7) and α-DTX (Fig. 3 D–F; n = 10). The 4-AP- and α-DTX-sensitive components of the depolarization-activated outward currents consisted of both slowly inactivating and steady state (as measured at 5 sec) components (Fig. 3). The depolarization-activated outward current that remained in the axon after bath application of 4-AP or α-DTX did not exhibit evidence of slow inactivation (Figs. 3B and E and 4B).
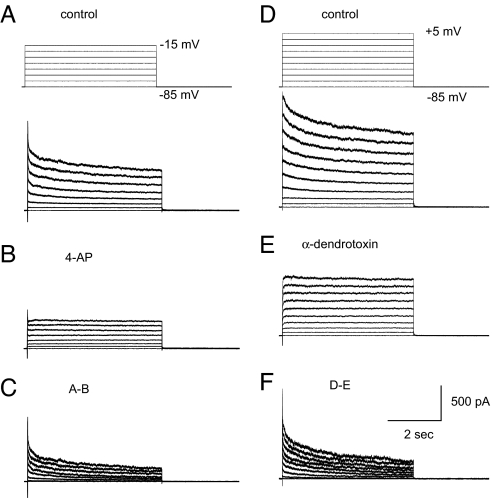
Fig. 3.
A slowly inactivating outward current in the axon is blocked by 4-AP and α-DTX. (A) Axonal (150 μm from soma) voltage steps activates a slowly inactivating outward current. (B) The slowly inactivating current is blocked by 4-AP (40 μM). (C) Subtraction reveals the 4-AP outward current to be rapidly activating, slowly inactivating, and containing a persistent component. (D) Axonal (110 μm) voltage steps in another cell. (E) α-DTX (100 nM) blocks the slowly inactivating current. (F) Subtraction reveals that α-DTX has effects similar to 4-AP.
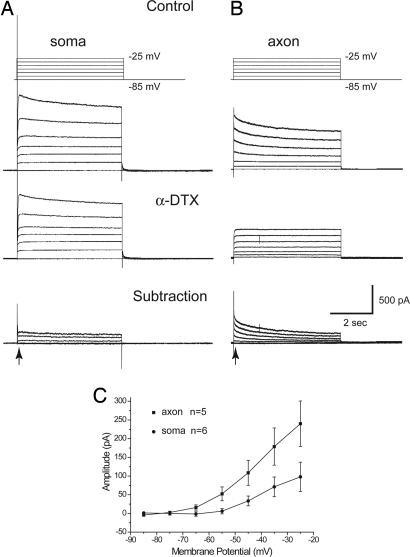
Fig. 4.
A slowly inactivating, 4-AP-sensitive, outward current is prominent in the axon but not the soma. (A) Voltage clamp steps from −85 to −25 mV applied to the soma results in the activation of outward currents, a component of which is blocked by α-DTX. (B) Axonal (120 μm from the soma) voltage clamp steps activate the typical slowly inactivating outward current that is blocked by α-DTX. (C) Plot of the voltage dependence and peak amplitude (as measured at the point illustrated by the arrows) of the α-DTX blocked outward currents, including the noninactivating component.
The 4-AP- and α-DTX-sensitive current activated rapidly (within 1 msec) and slowly inactivates with a time course that could be well fit (r2 = 0.95) by a single exponential function of 1.5 ± 0.5 s or even better fit (r2 = 0.99) by a double-exponential function with τs of 68 (± 10) msec and 1.44 ± 0.23 sec (not shown). These two time courses were not strongly voltage dependent over the voltage range of −75 to 0 mV (not shown; n = 10).
α-DTX selectively blocks K+ channels that contain either Kv1.1, 1.2, or 1.6 subunits (9, 22, 23), whereas low doses of 4-AP blocks Kv 1.1, 1.2, 1.5 and 3.1 subunits (9, 22). However, we found that application of 4-AP (40 μM) did not block any additional depolarization-activated outward current after the application of α-DTX [100 nM; n = 5; supporting information (SI) Fig. 6], indicating that the effects of 4-AP observed here are mediated largely or exclusively through α-DTX-sensitive channels. rTityustoxin-Kα is relatively selective for Kv 1.2 subunits (24), whereas dendrotoxin-K is relatively selective for Kv1.1 subunits (25). Bath application of rTityustoxin-Kα (50–100 nM) resulted in a strong suppression of the slowly inactivating K+ current (n = 6), with the additional application of α-DTX having only a small added block (n = 2; SI Fig. 7). In contrast, bath application of dendrotoxin-K (100 nM) had either no or mild suppressing effects on the slowly inactivating K+ current (n = 4), and the additional application of α-DTX resulted in a strong block of the residual slowly inactivating K+ current (n = 3; SI Fig. 7).
The 4-AP- and α-DTX-Sensitive K+ Current Is Prominent in the Axon but Not in the Soma.
The dramatic difference in the effect of α-DTX and 4-AP on spike duration in the soma versus the axon suggests that there may be substantial regional differences in the contribution of this current to depolarization-activated outward currents. Indeed, the bath application of α-DTX (n = 4) or 4-AP (n = 5) blocked a noninactivating K+ current in somatic recordings (Fig. 4A), with a relative lack of the large, slowly inactivating α-DTX- and 4-AP-sensitive K+ current that is seen in nearly all of our axonal recordings (Fig. 4B). Indeed, comparison of the amplitude and voltage dependence of the α-DTX-sensitive component in the soma and axon initial segment revealed a significantly larger amplitude in the axon (Fig. 4C), with a voltage threshold in both locations of approximately −65 to −70 mV. The 4-AP- or α-DTX-sensitive component was, on average, 46.3 ± 11.1% of the outward axonal currents activated by depolarization. Bath application of 4-AP (40 μM) had no consistent effect on the frequency of spike generation versus current injected (f-I) plot during the intrasomatic current injection (n = 6; not shown).
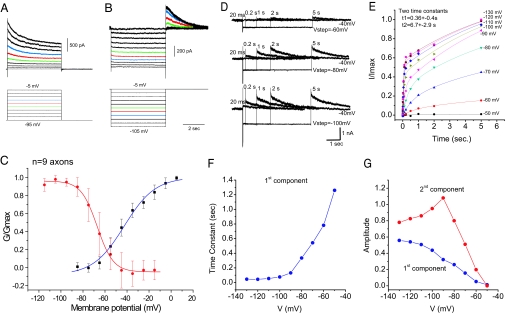
The Slowly Inactivating Axonal K+ Current Exhibits a Low Threshold.
Examination of the voltage dependence of the slowly inactivating outward current in axons revealed an activation threshold of approximately −70 to −75 mV and complete activation at −10 to 0 mV (Fig. 5C). Fitting the activation curve with a Boltzmann equation revealed a half-activation value of −43 mV and a slope of 14. The voltage dependence of steady-state inactivation of this current was examined through the application of 5-sec-long hyperpolarizing voltage steps and then examining the amplitude of the slowly inactivating outward current subsequently activated upon stepping back to −40 to −5 mV (Fig. 5B). Group data revealed that the current inactivated with a threshold of −90 to −100 mV, and inactivation was complete at −40 to −50 mV (Fig. 5C). Fits with a Boltzmann equation revealed values of half-inactivation of −67 mV and a slope of 7.3. Examination of the standard error of the mean bars (Fig. 5C) indicates that there is considerable variation between cells in the voltage dependence of inactivation. It is likely that these curves are broadened from the actual voltage dependence of activation and inactivation, owing to the limitations of single electrode voltage clamp applied to the cut end of a long cylindrical process such as an axon. However, our simulations suggest that this error may not be large (SI Figs. 8 and 9).
Fig. 5.
Properties of the slowly inactivating axonal outward current. (A) Axonal voltage steps from a holding potential of −95 mV. (B) Hyperpolarizing steps from a holding potential of −5 mV. (C) Construction of activation and inactivation curves for the slowly inactivating component (n = 9; average recording distance: 99±37 μm). (D) Removal of inactivation after steps to −60, −80, and −100 mV for varying lengths of time (axonal recording 300 μm from the soma). (E) Time course of normalized removal of inactivation data (n = 8; normalized to the tail current after a 5-sec step to −130 mV). Two time courses of removal of inactivation are found, with average time constants of 6.7 and 0.26 sec (average axonal recording distance: 224 ±55 μm). (F) The time constant of the fast component becomes slower with depolarization. (G) The contributions of the faster (first) and slower (second) components varied with voltage.
Recovery of Inactivation Is Slow and Voltage-Dependent.
The slow time course with which axonal spikes return to normal with hyperpolarization (Fig. 2) suggests that the current responsible may have a slow time course of removal of inactivation. To examine this possibility, we applied hyperpolarizing current steps of varying amplitudes and durations from a holding potential of −40 mV (Fig. 5 D–G). These voltage steps revealed at least two time courses for removal of inactivation (Fig. 5 D and E). At each membrane potential tested, the time course of removal of inactivation could be well fit (r2 > 0.98) by a combination of two exponential functions, with the first exponential function having an average value of 0.36 ± 0.4 s and the second having an average value of 6.7 ± 2.9 s. Examining the voltage dependence of these two exponential functions, however, revealed that the more rapid component may shorten significantly with increased hyperpolarization (Fig. 5F), whereas the second, slower component was less sensitive to membrane potential, having a value between 3.2 and 10.6 s at all levels (data not shown). The amplitude of both components increased with hyperpolarization of the axon, with the slow component being larger at all membrane potentials (Fig. 5G), and with the relative contribution of the slow-to-faster component peaking at approximately −90 mV. Because the time constant of the faster component shortened with hyperpolarization, the slowest removal of inactivation occurred at the most depolarized membrane potentials. In addition, at hyperpolarized membrane potentials, removal of inactivation exhibited a clear, two-component time course (Fig. 5E). Simulations suggest that the time constants of inactivation and removal of inactivation of the slowly inactivating K+ current are consistent with the slow changes in axonal spike duration observed with somatic depolarization and hyperpolarization (SI Figs. 9 and 10).
Discussion
We demonstrate here that cortical pyramidal cell axons exhibit a prominent, slowly inactivating K+ current that is blocked by α-DTX and low doses of 4-AP. This slowly inactivating current is responsible for axonal spike repolarization and controls axonal excitability. Voltage-dependent changes in this transient outward current contribute strongly to time- and voltage-dependent changes in axonal spike duration, which may have important functional implications for the operation of cortical networks and could be used as a mechanism for the intracortical “analog” transfer of information.
Rapidly activating K+ currents that slowly inactivate over a period of seconds and are blocked by low doses of 4-AP and α-DTX have been characterized in multiple cell types including hippocampal and cortical pyramidal cells (18–20, 26, 27). Because the activation of this type of current often occurs below spike threshold, they can cause a prolonged delay in the generation of the first spike, resulting in this class of current being called ID (18, 19). Similarly, here, we noted that depolarization of layer 5 pyramidal cell axons caused a slow depolarizing ramp that was blocked by 4-AP (Fig. 1). The rapid activation kinetics (<1 msec) of this current allows it to contribute strongly to the repolarization of axonal spikes. In fact, the strong prolongation of axonal spikes with low doses of 4-AP or α-DTX suggests that ID is the major current underlying spike repolarization in these intracortical axons. Hippocampal and cortical somatic spike duration can be prolonged by 4-AP or α-DTX, although typically to a much more modest degree then observed here for intracortical axons (21, 26, 28–31). The ability of 4-AP and/or α-DTX to prolong spikes is most prominent in neocortical neurons that generate short-duration spikes, such as fast spiking GABAergic interneurons or thin spiking pyramidal cells (21, 30, 31), suggesting that 4-AP- and α-DTX-sensitive K+ currents are especially important in the generation of short-duration spikes. There is considerable evidence that K+ channels containing Kv3.1 are also important for spike repolarization in fast spiking inhibitory interneurons (32).
A role for 4-AP- and α-DTX-sensitive K+ channels in controlling cortical axonal excitability has long been suggested by the observed ability of these agents to increase the evoked or spontaneous release of excitatory or inhibitory transmitters (13–15). Extracellular recordings of hippocampal axonal fiber potentials have observed increases in duration with application of low doses of 4-AP (14). In the cerebellum, simultaneous direct recordings of basket cell axon terminals and somata reveal a prominent role for slowly inactivating, 4-AP- and α-DTX-sensitive K+ currents in spike repolarization in the axon but not in the soma (33). At hippocampal mossy fiber terminals, an α-DTX-sensitive transient K+ current strongly controls activity-dependent changes in spike duration, although this current rapidly inactivates like an A-type K+ current. Prolonged depolarization or repetitive spike generation can result in inactivation of this current, leading to an increase in presynaptic spike duration, an increase in spike-dependent Ca2+ entry, and subsequent facilitation of evoked transmitter release (34). In some presynaptic terminals, such as at the calyx of Held in the medial nucleus of the trapezoid body (MNTB), 4-AP- and α-DTX-sensitive K+ currents strongly control axonal and terminal excitability, limiting responses to single spikes, without a strong contribution to spike duration (10).
α-DTX specifically blocks K+ channels that contain at least one α subunit that is Kv1.1, 1.2, or 1.6, whereas low doses of 4-AP appears to block channels containing these subunits as well as those containing Kv3.1. Tityustoxin-Kα, which is relatively specific for K+ channels containing Kv1.2 subunits (24) strongly blocked the slowly inactivating K+ current in cortical axons, whereas dendrotoxin-K, which is relatively specific for Kv1.1 subunits (25, 35), exhibited only marginal effects. Together, these results implicate K+ channels containing Kv1.2 subunits, either in a heteromeric or homomeric mixture (11, 36, 37) are the mediators of the D-current responsible for axonal spike repolarization. Immunocytochemical studies reveal the prominent expression of Kv 1.2 channels in axons and axonal terminal fields throughout the brain (6, 11, 38, 39). In the neocortex, Kv1.2 is of particularly high density in the more distal portions of the axon initial segment of cortical pyramidal cells (6). In addition to this localization, other axonal preparations have consistently revealed the prominent localization of Kv1 channels in the juxtaparanodal region of nodes of Ranvier (10, 39).
Previously, we demonstrated that prolonged depolarization of layer 5 somata results in a slow increase in spike duration in the main axon, even up to 300 μm distal (5). Here, we demonstrate that this effect is mediated by the inactivation and removal of inactivation of the axonal D-current. The ability of the D-current to control spike duration according to membrane potential may have a significant and important functional effect on synaptic transmission, particularly in the cerebral cortex, where many neurons form a dense local network of interconnections (40). We observed that small (10–15 mV) somatic depolarizations could have a significant enhancing effect on the amplitude of single-spike EPSPs between nearby cortical pyramidal cells, opening up the possibility that local cortical communication may operate in a mixed “digital” and “analog” mode (5). At least part of this depolarization-induced enhancement of synaptic transmission had a time constant that was similar to that observed here for changes in spike duration through the inactivation and removal of inactivation of ID (5), and previous studies have demonstrated that small increases in presynaptic spike duration can strongly enhance EPSC amplitude (34). It remains to be determined whether or not block of the slowly inactivating K+ currents results in an abolition of the ability of presynaptic somatic depolarization to enhance local excitatory synaptic transmission. Results obtained at the hippocampal mossy fiber synapse between dentate granule cells and CA3 pyramidal cells (4) as well as at the calyx of Held in the MNTB (41) indicate that changes in presynaptic spike generation may not be the major mechanism of depolarization-induced enhancement of transmitter release at these locations. Instead, both calcium-dependent, and non-calcium-dependent presynaptic mechanisms other than changes in spike duration have been implicated (4, 5, 41). Even so, our results indicate that a slowly inactivating, 4-AP-sensitive, K+ current is critical in the control of axonal spike duration, and therefore, by implication, in intracortical synaptic communication.
Materials and Methods
Slice Preparation.
Coronal slices from prefrontal cortex were prepared from 7- to 12-week-old ferrets and P17–22 Sprague–Dawley rats. The results obtained from both species were qualitatively similar and were therefore combined. Animals were initially deeply anesthetized with pentobarbital (30 mg/kg) and decapitated and slices formed as described (1). Slice solution contained 126 mM NaCl, 2.5 mM KCl, 2 mM MgSO4, 2 mM CaCl2, 26 mM NaHCO3, 1.25 mM NaH2PO4, and 25 mM dextrose (315 mOsm, pH 7.4), and the temperature was 35°C for incubation and 36°C–36.5°C for recording. Cortical neurons were visualized with an upright infrared-differential interference contrast (IR-DIC) microscope (BX51WI; Olympus, Melville, NY).
Electrophysiological Recordings.
Whole-cell recordings were achieved from both soma (see SI Methods) and the terminal bleb of the cut main axon of layer 5 regular spiking neurons by using a Multiclamp 700B amplifier (Axon Instruments, Union City, CA) as described (1, 5). Patch pipettes (5–6 MΩ for soma and 9–15 MΩ for the axon) were filled with 140 mM potassium gluconate, 3 mM KCl, 2 mM MgCl2, 2 mM Na2ATP, 10 mM Hepes, and 0.2 mM EGTA (pH 7.2 with KOH, 288 mOsm). Alexa Fluor 488 (100 μM) and biocytin (0.2%) were added to the pipette solution for tracing and labeling the recorded pyramidal cells. During recording, access resistance was monitored frequently and was compensated by up to 70%. Recordings with access resistance >25 MΩ for somatic recording or 45 MΩ for axonal recording, were discarded. Bridge balance and capacitance neutralization were carefully adjusted before and after every experimental protocol. The distance of the axonal recording site was measured after fixation and biocytin visualization. Action potential durations were measured at half-amplitude.
The cut end of the main axon, through which we obtained our axonal recordings, is, to some degree, an abnormal structure. We do not feel that this had a strong adverse effect on our results. Although the recordings will be, to some degree, influenced by the local properties of the bleb, the relatively small size (3–5 μm) of the terminal bleb in comparison with the 50–300 μm of the main axon to which it is attached indicates that the ionic currents and spike properties that we record are likely to be strongly dominated by those of the intact axon. Computational models have confirmed that changing the density of ion channels in this terminal bleb does not significantly affect the properties of the currents expected from whole-cell recordings from our axons (data not shown) (16). Finally, at subthreshold membrane potentials, we have measured the axonal length constant to be >400 μm (5), although the activation of K+ currents with depolarization will cause this to shorten significantly.
Measurements of Ionic Currents.
The slowly inactivating outward current was measured 100 msec after the onset of the depolarizing voltage step to avoid measurement of the rapidly inactivating K+ current, IA. The current remaining after ≈5 s (100 msec before the end of the depolarizing step) was taken as an estimate of the noninactivating component and was subtracted from the peak outward current, to yield a measure of the inactivating component. This inactivating component is completely blocked by 4-AP and α-DTX (Figs. 3 and 5), indicating that it is not necessary to apply these drugs to every cell to measure the 4-AP- and α-DTX-sensitive, slowly inactivating outward current. The voltage dependence of activation and inactivation of the slowly inactivating component was measured by estimating the conductance by using an ohmic relationship: G = I/(V − Vrev), where I is the amplitude of the inactivating component, V is the step potential, and Vrev is −107 mV, the estimated reversal potential for K+, given our internal and external ion solutions. The activation and inactivation curves were then normalized to the maximal conductance for each cell.
Data are presented as mean ± standard deviation in the text. Error bars in the illustrations are standard error of the mean. A liquid junction potential of 10 mV was subtracted from each recording (42, 43).
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (D.A.M.), the Howard Hughes Foundation, the Kavli Institute for Neuroscience, the 973 Program of China (2006CB806600), and Shanghai Commission of Science and Technology Grant 06dj14010.
Abbreviations
- EPSP
excitatory postsynaptic potential
- MNTB
medial nucleus of the trapezoid body.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702041104/DC1.
References
- 1.Shu Y, Duque A, Haider B, Yu Y, McCormick DA. J Neurophysiol. 2007;97:746–760. doi: 10.1152/jn.00922.2006. [DOI] [PubMed] [Google Scholar]
- 2.Stuart G, Schiller J, Sakmann B. J Physiol. 1997;505(Pt 3):617–632. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Debanne D. Nat Rev Neurosci. 2004;5:304–316. doi: 10.1038/nrn1397. [DOI] [PubMed] [Google Scholar]
- 4.Alle H, Geiger JR. Science. 2006;311:1290–1293. doi: 10.1126/science.1119055. [DOI] [PubMed] [Google Scholar]
- 5.Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Nature. 2006;441:761–765. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- 6.Inda MC, DeFelipe J, Munoz A. Proc Natl Acad Sci USA. 2006;103:2920–2925. doi: 10.1073/pnas.0511197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komada M, Soriano P. J Cell Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astman N, Gutnick MJ, Fleidervish IA. J Neurosci. 2006;26:3465–3473. doi: 10.1523/JNEUROSCI.4907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, et al. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 10.Dodson PD, Forsythe ID. Trends Neurosci. 2004;27:210–217. doi: 10.1016/j.tins.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Southan AP, Robertson B. J Neurosci. 1998;18:948–955. doi: 10.1523/JNEUROSCI.18-03-00948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu Y, Ge SY, Ruan DY. Brain Res. 2004;1006:225–232. doi: 10.1016/j.brainres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Qian J, Saggau P. J Neurophysiol. 1999;81:288–298. doi: 10.1152/jn.1999.81.1.288. [DOI] [PubMed] [Google Scholar]
- 15.Lambe EK, Aghajanian GK. J Neurosci. 2001;21:9955–9963. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormick DA, Shu Y, Yu Y. Nature. 2007;445:E1–E2. doi: 10.1038/nature05523. discussion E2–E3. [DOI] [PubMed] [Google Scholar]
- 17.Palmer LM, Stuart GJ. J Neurosci. 2006;26:1854–1863. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storm JF. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- 19.Storm JF. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- 20.Wu RL, Barish ME. J Neurosci. 1992;12:2235–2246. doi: 10.1523/JNEUROSCI.12-06-02235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Zhang JJ, Hu GY, Wu CP. Neuroscience. 1996;73:57–68. doi: 10.1016/0306-4522(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 22.Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 23.Harvey AL, Robertson B. Curr Med Chem. 2004;11:3065–3072. doi: 10.2174/0929867043363820. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins WF. J Pharmacol Exp Ther. 1998;285:1051–1060. [PubMed] [Google Scholar]
- 25.Robertson B, Owen D, Stow J, Butler C, Newland C. FEBS Lett. 1996;383:26–30. doi: 10.1016/0014-5793(96)00211-6. [DOI] [PubMed] [Google Scholar]
- 26.Foehring RC, Surmeier DJ. J Neurophysiol. 1993;70:51–63. doi: 10.1152/jn.1993.70.1.51. [DOI] [PubMed] [Google Scholar]
- 27.Guan D, Lee JC, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. J Physiol. 2006;571:371–389. doi: 10.1113/jphysiol.2005.097006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bekkers JM, Delaney AJ. J Neurosci. 2001;21:6553–6560. doi: 10.1523/JNEUROSCI.21-17-06553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bossu JL, Capogna M, Debanne D, McKinney RA, Gahwiler BH. J Physiol. 1996;495(Pt 2):367–381. doi: 10.1113/jphysiol.1996.sp021600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, McBain CJ. J Physiol. 1995;488(Pt 3):661, 672. doi: 10.1113/jphysiol.1995.sp020998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou FM, Hablitz JJ. J Neurophysiol. 1996;76:651–667. doi: 10.1152/jn.1996.76.2.651. [DOI] [PubMed] [Google Scholar]
- 32.Rudy B, McBain CJ. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 33.Southan AP, Robertson B. J Neurosci. 2000;20:114–122. doi: 10.1523/JNEUROSCI.20-01-00114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geiger JR, Jonas P. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 35.Wang FC, Parcej DN, Dolly JO. Eur J Biochem. 1999;263:230–237. doi: 10.1046/j.1432-1327.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes KJ, Keilbaugh SA, Barrezueta NX, Lopez KL, Trimmer JS. J Neurosci. 1995;15:5360–5371. doi: 10.1523/JNEUROSCI.15-07-05360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheng M, Liao YJ, Jan YN, Jan LY. Nature. 1993;365:72–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- 38.Monaghan MM, Trimmer JS, Rhodes KJ. J Neurosci. 2001;21:5973–5983. doi: 10.1523/JNEUROSCI.21-16-05973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trimmer JS, Rhodes KJ. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- 40.Binzegger T, Douglas RJ, Martin KA. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awatramani GB, Price GD, Trussell LO. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 42.Fricker D, Verheugen JA, Miles R. J Physiol. 1999;517(Pt 3):791–804. doi: 10.1111/j.1469-7793.1999.0791s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neher E. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.