Abstract
We previously reported that long-term cyclic estrogen (E) treatment reverses age-related impairment of cognitive function mediated by the dorsolateral prefrontal cortex (dlPFC) in ovariectomized (OVX) female rhesus monkeys, and that E induces a corresponding increase in spine density in layer III dlPFC pyramidal neurons. We have now investigated the effects of the same E treatment in young adult females. In contrast to the results for aged monkeys, E treatment failed to enhance dlPFC-dependent task performance relative to vehicle control values (group young OVX+Veh) but nonetheless led to a robust increase in spine density. This response was accompanied by a decline in dendritic length, however, such that the total number of spines per neuron was equivalent in young OVX+Veh and OVX+E groups. Robust effects of chronological age, independent of ovarian hormone status, were also observed, comprising significant age-related declines in dendritic length and spine density, with a preferential decrease in small spines in the aged groups. Notably, the spine effects were partially reversed by cyclic E administration, although young OVX+Veh monkeys still had a higher complement of small spines than did aged E treated monkeys. In summary, layer III pyramidal neurons in the dlPFC are sensitive to ovarian hormone status in both young and aged monkeys, but these effects are not entirely equivalent across age groups. The results also suggest that the cognitive benefit of E treatment in aged monkeys is mediated by enabling synaptic plasticity through a cyclical increase in small, highly plastic dendritic spines in the primate dlPFC.
Keywords: aging, estradiol, hormone, neocortex, plasticity
Numerous studies have demonstrated that E affects synaptic structure and function in multiple brain regions important for memory and cognition (1). In the hippocampus of young female rats, 17β-estradiol [the dominant estrogen (E) in rats, monkeys, and humans] increases the density of dendritic spines and axospinous synapses on CA1 pyramidal cells (2, 3). These effects are N-methyl-d-aspartate (NMDA) receptor-dependent (4), and E both directly increases NMDA receptor levels and facilitates NMDA receptor-mediated responses in CA1 pyramidal neurons (5, 6). E also affects GABAergic (7) and cholinergic (8) systems in the CA1 field of the young female rat hippocampus. The data from young adult monkeys are generally consistent with these observations, which is particularly relevant to humans given the reported similarities in cyclicity and menopause between women and female rhesus monkeys (9, 10). Of particular note, E increases CA1 spine number in ovariectomized (OVX) young African green (11) and rhesus monkeys (12). Estrogen administration also increases spine number in layer I of dorsolateral prefrontal cortex (dlPFC) of young rhesus monkeys (13) and enhances cholinergic and monoaminergic inputs to this region (14, 15), demonstrating that potential targets for ovarian hormone influences on cognitive function extend beyond the hippocampus in primates.
Given the vulnerability of many of these same systems to aging, together with the marked decline in circulating ovarian hormones at menopause in women, a number of laboratories have investigated potential influences of endocrine senescence on cognitive and neurobiological aging in animal models. Studies comparing young and aged OVX rats have demonstrated that the hippocampal response to E in aged female rats is attenuated as compared with young females with respect to both CA1 synapse number (16) and cognitive function (17), and that the efficacy of intervention declines further when the delay between OVX and initiation of E administration is extended for several months (18, 19). However, the emerging story in aged non-human primates differs in important ways from the rodent findings (20). First, the available evidence suggests that the cognitive benefit of E in aged OVX monkeys is substantially greater than in young adults, particularly on tests emphasizing dlPFC integrity (21). A previous study, for example, reported that performance on the dlPFC-dependent delayed response (DR) is not enhanced by continuous E treatment in OVX young monkeys (22). In contrast, the same group observed a reliable cognitive benefit of intervention in middle-aged monkeys (23). Extending the latter results, we recently reported that long-term cyclic E substantially improves DR performance in aged OVX monkeys, yielding task accuracy comparable to levels observed in young adults (24). The same E-treated monkeys also displayed a corresponding increase in spine density, together with a subtle shift toward smaller spines on dlPFC neurons (i.e., area 46) (25), establishing a potential neurobiological substrate for the cognitive benefit of E.
The present study extended our analysis of estrogen influences on the cognitive and morphological outcome of aging to young adult monkeys treated equivalently. The results revealed both age and treatment effects, suggesting that whereas the aged dlPFC remains responsive, the specific pattern is qualitatively and quantitatively different in young monkeys. Overall, the findings suggest that whereas young adults without E can sustain excellent cognitive function against a background of dynamic spine plasticity, the “double hit” of age and E deficiency leads to morphologic alterations that significantly compromise the functional integrity of dlPFC in aged female rhesus monkeys.
Results
Estrogen Levels.
The same cyclical estradiol treatment that was shown previously to elevate serum levels to a physiological range, consistent with the preovulatory peak in intact monkeys (24), was used in the young E treated animals. Serum samples taken at the time of perfusion (24 h after the final injection) demonstrated serum estradiol levels averaging 112 pg/ml in the treatment group and 24 pg/ml in the Veh group (P < 0.01). Excluding an unexplained, outlying value in the Veh group, serum estradiol levels among controls averaged 15 pg/ml.
DR Performance.
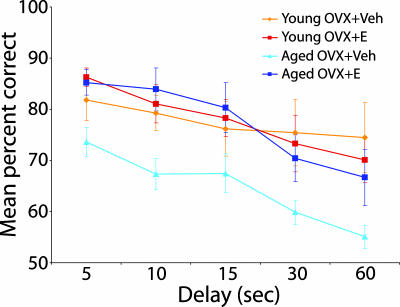
We previously reported that cyclic E treatment substantially improves DR performance in aged OVX monkeys (24), establishing a valuable basis for comparison with the current findings from young subjects, tested under identical conditions. Although the number of trials required to reach the criterion level of accuracy with a 1-s delay was substantially higher in the aged Veh group, task acquisition at both the 0- and 1-s delay failed to differ reliably across age and treatment conditions (all P values > 0.5, data not shown). Notably, all subjects scored at comparable, high levels of accuracy (90% correct or better) before testing with longer retention intervals. Performance on the delay portion of the task, imposing successively more challenging memory demands, is illustrated in Fig. 1. Accuracy declined in all groups across delays of 5–60 s, confirming that the procedure effectively taxed memory (main effect of delay; P < 0.0001). Accuracy differed reliably across conditions (main group effect; P = 0.05), and there was no interaction between group and delay (P > 0.5). Subsequent between group contrasts demonstrated that the aged OVX+Veh group scored less accurately than monkeys in each of the other conditions (all P values < 0.03). Performance across the remaining groups, by comparison, was statistically equivalent (all P values > 0.9). These results confirm our earlier conclusion that cyclic E treatment substantially reverses DR impairment in the aged monkey, demonstrating for the first time that accuracy is restored to levels comparable to young OVX subjects. In addition, the findings indicate that, in striking contrast to the effects observed in aged subjects, spatiotemporal memory measured by the DR task appears insensitive to OVX and E treatment in young adult monkeys.
Fig. 1.
Performance on DR of young and aged OVX+Vehicle (Veh) and OVX+E monkeys. Unlike aged monkeys, young OVX+Veh and young OVX+E rhesus monkeys performed equally well on DR tasks. In addition, the performance of the aged OVX+E group was equivalent to both young treatment groups, with the aged OVX+Veh performing significantly worse than all three other age and treatment groups. Error bars represent SEM.
Effects of Long-Term Cyclic E Treatment on Dendritic Arbor of Area 46 Layer III Pyramidal Neurons.
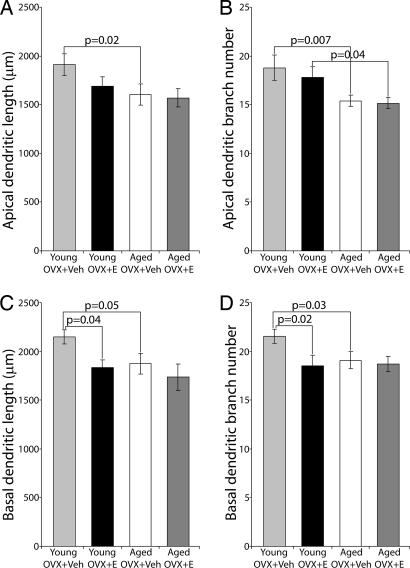
We previously reported that long-term cyclic E treatment had no effect on total length and branching pattern of dendrites in layer III pyramidal neurons of area 46 in aged female rhesus monkeys (25). In contrast, as illustrated in Fig. 2, a numerically substantial treatment effect was apparent in young female rhesus monkeys, with basal dendritic length and branch number in the young OVX+Veh group exceeding that of young OVX+E (mean ± SEM, total basal dendritic length 2,148.3 ± 72.5 μm and branch number of 21.5 ± 0.7 in OVX+Veh compared with total basal dendritic length of 1,832.8 ± 81.5 μm and branch number of 18.5 ± 1.1 in young OVX+E; P = 0.04 for total basal dendritic length, P = 0.02 for basal branch number). A similar trend observed among apical dendrite parameters failed to reach significance. Sholl analysis showed that the relative increase in basal dendritic arbor in young OVX+Veh compared with E treated adults occurred mainly 60–120 μm from the soma (data not shown).
Fig. 2.
Quantitative analysis of aging and treatment effects on dendritic arbor of layer III pyramidal neurons in area 46. Significant age-related dendritic arbor reductions were manifested between young and aged OVX+Veh groups on apical (A and B) and basal (C and D) dendritic length and branch number. An age-related decrease in apical branch number also occurs between young and aged OVX+E groups (B). Young OVX+Veh monkeys demonstrated increased dendritic length and higher branch number when compared with young OVX+E monkeys, although significance was limited to basal dendrites (C and D). Error bars represent SEM.
Comparison of the young groups with our previous data from aged animals revealed clear effects of aging on dendritic arbor of layer III pyramidal neurons in area 46. Apical and basal dendritic length and branch number (Fig. 2) were decreased in the aged monkeys, and these effects were especially pronounced in comparisons between the young OVX+Veh (total dendritic length 1,911.3 ± 112.2 μm for apical and 2,148.3 ± 72.5 μm for basal; total branch number, 18.8 ± 1.3 for apical and 21.5 ± 0.7 for basal) and aged OVX+Veh groups (total dendritic length 1,603.4 ± 107.9 μm for apical and 1,875.0 ± 107.2 μm for basal; total branch number, 15.4 ± 0.6 for apical and 19.1 ± 0.9 for basal). Additional, marginally significant age-related decreases in apical branch number were observed between the young OVX+E and aged OVX+E groups (P = 0.04). Sholl analysis demonstrated that the aging effects occurred mainly 60–150 μm from the soma in the apical dendritic arbor (data not shown).
Effects of Long-Term Cyclic Treatment with 17β-Estradiol on Dendritic Spine Density.
As previously observed in aged OVX female rhesus monkeys (25), young OVX female rhesus monkeys also display a profound treatment effect on spine density, with E treatment increasing apical (from 1.06 ± 0.04/μm to 1.31 ± 0.04/μm) and basal dendritic spine density (from 1.03 ± 0.03/μm to 1.25 ± 0.04/μm) across all dendritic orders except the primary dendrites (Fig. 3, P < 0.001 for both apical and basal dendrites). Comparisons across age groups in the same treatment conditions (i.e., young versus aged Veh groups, young versus aged E groups) revealed highly reliable declines in spine density in both apical and basal dendrites (Fig. 3, P < 0.001). Importantly, E treatment partially restored spine density among aged subjects, resulting in levels comparable to vehicle-treated young adults, but substantially lower than in young monkeys provided E.
Fig. 3.
Quantitative analysis of aging and treatment effects on spine density of layer III pyramidal neurons in area 46. Significant treatment effects are seen in both young and aged spine densities in apical (A) (average from 2nd to 4th order) and basal (B) dendrites (P < 0.001 for both young and aged groups). Significant age-related decreases are evident in spine densities on both apical (A) and basal (B) dendrites (P < 0.001 for young and aged OVX+Veh and OVX+E groups). Error bars represent SEM.
We were intrigued by the opposing effect of treatment in young animals on dendritic length and spine density. Notably, the 18.3% decrease in spine density in young OVX+Veh animals, relative to the OVX+E group, was coincident with a 15.3% increase in dendritic length and as a consequence, spine number per neuron was roughly equivalent across these groups. These findings suggest that total dendritic spine representation is held constant under conditions of low and high circulating E in the young adult dlPFC, potentially accounting for the lack of treatment effect on DR performance. In contrast, dendritic length appears insensitive to estrogen treatment in aged monkeys, and fails to counteract the robust spine proliferation induced by hormone administration.
Effects of Long-Term Cyclic 17β-Estradiol Treatment on Dendritic Spine Morphology in Area 46 Layer III Pyramidal Neurons.
Current evidence indicates that spine size (e.g., spine head diameter, head volume and surface area) is proportional to synapse size (26) and related to glutamate receptor representation (27, 28). In addition, small spines appear especially motile and plastic (29, 30), potentially comprising a specialized subpopulation, distinguished by its capacity for growth and expansion in relation to new learning (29). We previously investigated the effects of E on spine head diameter (Hd), head length (Hl), and neck length (Nl) in aged OVX rhesus monkeys and demonstrated that E led to both an increase in spine density and a shift toward smaller spines (25). The current analysis determined whether there was an overall E-induced shift in the spine size distribution, or a disproportionate increase in the incidence of spines with small or large heads in young OVX monkeys. In addition, we performed analyses comparing all four groups to reveal any age effects on spine size. The results for these spine parameters are presented in Fig. 4, displayed as cumulative percentage against increasing size. The effect of age on spine head diameter (Fig. 4A) was dramatic and highly significant (P < 0.0001), with aged animals displaying far fewer small spines than young monkeys. Hormone treatment exerted an opposing effect; i.e., E administration induced a leftward shift in the size distribution, toward small spines. The latter effect, however, failed to fully reverse the result attributable to age. Although E administration shifted size to the left in both age groups, this effect was more pronounced in aged monkeys (P < 0.005) than in young subjects (P = 0.02).
Fig. 4.
Cumulative frequency analysis of aging and treatment effects on spine size: Hd, Hl, and Nl. (A) Significant aged related increases of Hd were observed between the aged (OVX+Veh and OVX+E) vs. young (OVX+Veh and OVX+E) groups (P < 0.0001). Although a highly significant treatment effect was observed in the aged group (P < 0.005), i.e., Hd decreased following treatment, a marginally significant difference was observed in young animals (P = 0.02). (B) No aging and treatment effects on Hl were observed. (C) Overall, aging significantly reduced Nl (P < 0.0001). Although no treatment effects on Nl were observed within the aged group, a marginally significant increase in Nl was observed in the young OVX+E compared with young OVX+Veh group (P = 0.015).
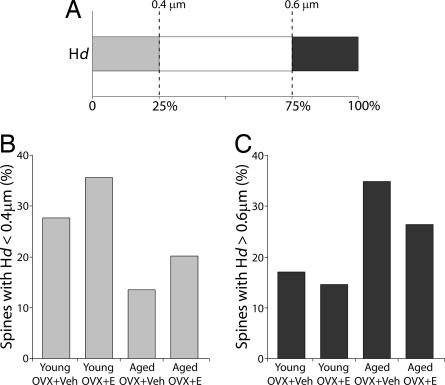
A further, quartile analysis confirmed that E induced a shift toward smaller dendritic spines in both young and aged monkeys (P < 0.001, Fig. 5). Independent of hormone treatment condition, it is also noteworthy that whereas spines in the 25th percentile were nearly twice as prevalent in young monkeys, large spines (75th percentile) clearly predominated in the aged group. Perhaps most striking, small spines in the young OVX+E group were nearly 3-fold more frequent than among aged OVX+Veh monkeys, reinforcing the heightened vulnerability of aged animals without E. Head length, by comparison, was similar across groups, without discernible treatment or age effects (Fig. 4B).
Fig. 5.
Quartile analysis of Hd. (A) Randomly sampled spines (850; 170 in each of five animals) were selected from each of the four groups to determine the relative contribution of each group to the smallest 25th percentile (<0.4 μm) and the largest 25th percentile (>0.6 μm). Proportions of spines below and above the two quartiles were generated for each group. (B) The smaller (Hd < 0.4 μm) spine counts in the young animals were almost double those of the aged animals (P < 0.001). (C) The larger (Hd > 0.6 μm) spine counts were higher in the aged animals (P < 0.001). Overall, E treatment induces greater numbers of smaller spines in both young and aged groups (P < 0.001).
Neck length measurements also revealed prominent effects of age, together with a more subtle influence of hormone status (Fig. 4C). Aged monkeys displayed a strong shift to the left in cumulative percentage, demonstrating a preponderance of short spines, and corresponding loss of long spines, as compared with young monkeys (P < 0.0001). E treatment had no effect on neck length in aged monkeys and therefore failed to account for the observed age-related shift toward shorter spines. In contrast, E treatment induced a marginally significant increase in long spines in young animals (P = 0.015).
Discussion
Our results offer a framework for understanding the cognitive resiliency of young female rhesus monkeys under conditions of fluctuating ovarian hormone levels, and the contrasting vulnerability of aged female rhesus monkeys with chronically low E. In the morphological analysis, we focus on three reflections of neuronal and synaptic structure and plasticity: dendritic arbor, spine number, and spine size. All three indices display substantial age effects that could impact prefrontal cortex function, but they vary in the degree to which E treatment counteracts the influence of aging. In addition, the data on dendritic arbor and spine density from young animals suggest that the effect of treatment on one neuronal attribute can be neutralized by its opposing influence on another structural parameter. Focusing exclusively on layer III pyramidal neurons in area 46 of the monkey prefrontal cortex, the present results provide substantial, novel insight concerning the effects of neuroendocrine aging on a class of neurons that directly mediate high level cognitive processes such as working memory (31) and that support critically important corticocortical interactions with other association areas (32, 33).
Aging affects all three parameters investigated: (i) dendritic length and number of branches decreases with age; (ii) spines are lost throughout the dendritic tree; (iii) age-related spine loss is particularly pronounced among long spines with small heads that are thought to disproportionately participate in dynamic synaptic plasticity. Age-associated decreases in dendritic length and branch number are insensitive to E treatment. Furthermore, in young animals, the absence of E is associated with increased dendritic length and branch number, particularly in the basal tree. In contrast to dendritic arbor, E increases spine density in both young and aged OVX monkeys. Thus, the combined effects of aging and E deficiency comprise a double hit in aged OVX+Veh monkeys. The E-induced recovery in aged monkeys is partial, returning spine density to the level of Veh-treated young adults, but still substantially less than observed in young monkeys given E. Importantly, the E-induced increase in spine density in young monkeys occurs alongside a concurrent decrease in dendritic length, leading to an equalization of total spine number per neuron with and without E. As with spine density, aging and E treatment also exert opposing influences on spine size, with the effect of age predominating. Specifically, aging is associated with a shift in the distribution of spine size in which long neck, small head spines are lost or do not form. E administration only partially reverses this effect, and the full extent of vulnerability was particularly striking in comparisons between young OVX+E monkeys and aged monkeys that received vehicle injections; here, the young adult group displayed 3-fold more spines with small heads than aged subjects. The present results reveal a complex interplay of age and E effects on dendritic architecture that provides a potential substrate for ovarian hormone influences on cognitive function mediated by prefrontal cortex. We previously reported that cyclic E treatment profoundly benefits DR performance in aged female monkeys, restoring accuracy to levels statistically equivalent with intact young adult rhesus monkeys (24). Here we extended those findings to conduct a parallel assessment in OVX young adults. The key results were that E treatment had no effect on DR performance in young monkeys, and in contrast, that cyclical E administration fully reversed the marked impairment observed in vehicle-treated aged subjects. These findings are consistent with earlier reports demonstrating little or no cognitive effect of E in young monkeys (21, 34), and importantly, they are the first to document a corresponding morphologic resiliency in the young primate prefrontal cortex. First, increased dendritic arbor in young untreated monkeys largely counterbalances E-induced increases in spine density, as gauged by the total number of spines per neuron. Second, and perhaps more important, the relative abundance of small spines in both treated or untreated young monkeys exceeded that of treated aged monkeys, suggesting that formation and retention of small spines is less dependent on E in young animals and remains robust in the absence of E.
Spine size may be a critically important index of the capacity for synaptic plasticity in the aged brain. Available evidence suggests that dynamic functional and structural plasticity are preferentially mediated by small, thin spines with a relatively long neck, and that active spinogenesis is selective for this morphological class (29, 30). Rodent studies also suggest that these spines exhibit expansion, synaptic stabilization and retraction (29, 30, 35), although the prevalence of these events throughout different neocortical areas is controversial (36). Nonetheless, there is reason to suspect that small “learning spines” (29) may play a particularly significant role under testing conditions, like DR, that place substantial demands on working memory, i.e., the capacity for rapidly updating acquired information (37). Thus, the dramatic loss of these spines in aged monkeys lacking E treatment may be a critically important contributor to the robust deficits they exhibit on dlPFC-dependent cognitive tasks. Viewed in relation to the behavioral and morphologic results from young adults, our proposal is that the representation of small, plastic spines in aged OVX monkeys without E may fall below a critical threshold needed to sustain normal learning mediated by the dlPFC. By this perspective, the influence of endocrine decline on cognitive aging is best understood as the cumulative outcome of declining synaptic plasticity and the reduced capacity for structural reorganization enabled by circulating E.
It remains to be determined whether learning-related spine plasticity and turnover are dependent on the specific timing of and schedule of E treatment in monkeys. In the present experiments, young and aged animals were treated cyclically, with an intramuscular injection of estradiol every 21 days throughout behavioral testing, and monkeys were killed 24 h after the last injection. The cyclical nature of E-induced spine formation has been demonstrated in rodent hippocampus (2), and it may be that cyclical E is critical for the proliferation of plastic spines that participate in encoding new learning. Furthermore, our OVX and subsequent treatment occurs in the peri-menopausal stage for the aged rhesus monkeys such that the cyclical exposure and resultant spinogenesis would not have been interrupted by an extended menopausal phase. The time of initiation of E treatment relative to menopause has become a major focus of research in women (38, 39), consistent with emerging findings in rats (40). The present study speaks to this issue indirectly, demonstrating that early intervention in aged primates can significantly benefit the structural and functional integrity of a key neocortical region that is vulnerable to aging. The dissection of age and neuroendocrine influences on the prefrontal cortex in primates will continue to be a high priority for research aimed at promoting optimally healthy cognitive aging.
Materials and Methods
Animals and Treatment.
All experiments were conducted in compliance with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
Sixteen young female Rhesus monkeys (Macaca mulatta; mean age ± SEM, 10 years ± 3 months) were used in the behavioral phase of this study, and 12 of the 16 were used in the morphologic analysis to demonstrate the treatment effects of long-term cyclical E treatment on layer III pyramidal cells in dlPFC (i.e., area 46) of young female rhesus monkeys. The protocols were identical to those used previously on aged female rhesus monkeys (24, 25), and data from all four age/treatment groups are used for new analyses in this report to reveal age and treatment effects. The young monkeys received bilateral OVX and were assigned to age-matched OVX+Veh and OVX+E treatment groups, as was done previously with the aged monkeys. After an average post-OVX interval of 29 weeks, OVX+E monkeys received estradiol cypionate (100 μg/ml of sterile peanut oil, i.m.; Amersham Pharmacia, Peapack, NJ) in a single injection every three weeks. OVX+Veh age-matched monkeys were provided an equivalent volume of vehicle injection according to the same schedule. Treatment extended over ≈2 years of behavioral testing. E and Veh injections were coded and administered in a blinded fashion until all experiments were completed. Serum estradiol values were measured at perfusion, 24 h after the final injection.
DR Testing.
The DR testing was equivalent to the aged animals tested in ref. 24. Subjects watched from behind a transparent screen while one of the wells of the apparatus was baited with a food reward, and both wells were then covered. During initial training, the screen was raised immediately, allowing monkeys to displace the cover and retrieve the reward if the baited location was chosen. Testing continued until animals met a criterion of 90% correct or better across nine consecutive blocks of 10 trials. In subsequent testing, a 1-s retention interval was imposed between baiting and the opportunity to respond. Training with a 1-s delay continued to the same performance criterion (≥90% correct over 90 trials), and the demands of testing were then made progressively more challenging by imposing successively longer retention intervals of 5, 10, 15, 30, and 60 s. Each delay was tested for a total of 90 trials (30 trials/daily session; intertrial interval = 20 s).
Quantitative Analyses of Dendritic Arbor, Spine Density and Spine Morphology.
Animals were perfused 24 h after the last E or Veh treatment, and dissected into multiple standardized blocks, one of which contained all of area 46. Sections (400-μm-thick) spaced 2.6 mm apart throughout the prefrontal cortex were collected for intracellular injections and quantitative analysis according to methods described in ref. 25. Layer III pyramidal neurons in Brodmann area 46 within dlPFC were targeted for analysis. Briefly, dendritic arbor from Lucifer Yellow filled pyramidal neurons [5–17 per monkey, total of 267 neurons (Fig. 6)] were reconstructed in 3D, using Neurolucida morphometry software (MicroBrightField, Williston, VT). Dendrograms and 3-D Sholl analyses were prepared for each neuron with NeuroExplorer. Dendritic arbor was expressed in terms of total dendritic length and total branch number.
Fig. 6.
Representative layer III pyramidal neuron of area 46. (A and B) LY-loaded neuron at intermediate magnification (B) was reconstructed by using Neurolucida and a 3D rendering dataset of the traced neuron (A) generated by NeuroExplorer was used for dendritic arbor measurements and to obtain the systematic sampling for spine density analysis (see text for details). (C) Deconvolved confocal z-stacks from the LY-loaded neuron for 3D spine density analysis. (D) Single spines were analyzed for Hd, Hl, and Nl. (Scale bars: A and B, 50 μm; C, 5 μm; D, 0.5 μm.)
Printed datasets of dendritic tree maps were used to allow identification of dendritic segments chosen in a systematic-random way for spine analysis. A clear acetate sheet, containing concentric rings, was placed over the working maps and oriented such that the cell soma and innermost rings were aligned over each other. Points of intersection between dendritic branches and the circles (60 μm apart) were used to identify those segments to be used for microscopic spine analysis. Confocal z-stacks of identified intersections were captured on a Zeiss LSM 410 (Carl Zeiss, Oberkochen, Germany). An average of 12 stacks were captured per neuron (total 189 neurons). The confocal stacks were deconvolved with AutoDeblur (Media Cybernetics, Silver Spring, MD) and imported to Neurolucida for 3D spine density, and to VIAS (41) for spine morphology analysis. All spine measurements were done manually (total 15,000 spines) from the z-stacks. Spine Nl was measured as the distance from point of attachment of the dendrite to the beginning of spine head. Hl was the distance across the spine in continuity with the spine neck. Hd was taken at the largest diameter perpendicular to the head/neck axis.
Statistical Analysis.
Group differences in serum estradiol levels in young monkeys were analyzed with a nonparametric (Mann–Whitney) test. Differences in behavioral performance across the four groups on DR were assessed by using repeated measures ANOVA and Fisher's probable least-squares difference. To assess possible differences in various morphometric parameters between groups, ANOVA, or, when appropriate, the nonparametric Kruskal–Wallis test, was applied. The values are shown as means ± SEM, calculated based on one aggregate (e.g., average) per animal. The two-way mixed model for repeated measures was used to test the treatment effect on dendritic length and branch number across apical and basal dendritic trees, and on spine density across basal and apical trees. For spine morphology measurements, the overall distribution difference between groups was examined by using the Kolmogorov–Smirnov test. The χ2 test was used for the quartile analysis of Hd. The overall statistical significance level was set at 0.05. For the pairwise comparisons of age and treatment effects, the P value threshold was corrected for multiple comparison (Bonferroni), and set at P = 0.0125. While adopting this conservative criterion, P values between the uncorrected 0.05 level and 0.0125 were considered indicative of potentially important observations, characterized as “marginally significant.” Statistical analyses were performed by using the software packages StatView and SAS (Version 9).
Acknowledgments
We thank Athena Wang, Carine Hamo, Ginelle Andrews, Susan Fink, Anne Canfield, Don Canfield, Mary Roberts, Sania Fong, Deborah Kent, Harry Arwell, Sona Santos, and Heather McKay for technical assistance; Douglas Ehlenberger and Alfredo Rodriguez for software development; and Drs. Murat Yildirim, Chet Sherwood, Anne Rocher, Jason Radley, Yong Tang, Christina Weaver, and Doron Kabaso for critical discussion. This work was supported by National Institutes of Health Grants AG16765, AG06647, AG10606, MH58911, MH60734, and RR16754.
Abbreviations
- dlPFC
dorsolateral prefrontal cortex
- E
estrogen
- OVX
ovariectomized
- Veh
vehicle
- DR
delayed response.
Footnotes
The authors declare no conflict of interest.
References
- 1.McEwen B. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 2.Woolley CS, McEwen BS. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould E, Woolley CS, Frankfurt M, McEwen BS. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolley CS, McEwen BS. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy DD, Cole NB, Greenberger V, Segal M. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbs RB. Horm Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- 9.Downs JL, Urbanski HF. Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- 10.Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- 11.Leranth C, Shanabrough M, Redmond DE., Jr J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- 12.Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, et al. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, et al. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- 14.Kritzer MF, Kohama SG. J Comp Neurol. 1999;409:438–451. doi: 10.1002/(sici)1096-9861(19990705)409:3<438::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Tinkler GP, Tobin JR, Voytko ML. J Comp Neurol. 2004;469:507–521. doi: 10.1002/cne.11028. [DOI] [PubMed] [Google Scholar]
- 16.Adams MM, Shah RA, Janssen WG, Morrison JH. Proc Natl Acad Sci USA. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savonenko AV, Markowska AL. Neuroscience. 2003;119:821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 18.Gibbs RB. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 19.Adams MM, Oung T, Morrison JH, Gore AC. Exp Neurol. 2001;170:345–356. doi: 10.1006/exnr.2001.7716. [DOI] [PubMed] [Google Scholar]
- 20.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacreuse A. Neuroscience. 2006;138:859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Voytko ML. Behav Neurosci. 2000;114:1078–1087. [PubMed] [Google Scholar]
- 23.Tinkler GP, Voytko ML. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:423–431. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Rapp PR, Morrison JH, Roberts JA. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris KM, Stevens JK. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- 28.Kharazia VN, Weinberg RJ. J Comp Neurol. 1999;412:292–302. doi: 10.1002/(sici)1096-9861(19990920)412:2<292::aid-cne8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 30.Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Goldman-Rakic PS. Ann NY Acad Sci. 1999;868:13–26. doi: 10.1111/j.1749-6632.1999.tb11270.x. [DOI] [PubMed] [Google Scholar]
- 32.Hof PR, Morrison JH. J Comp Neurol. 1995;352:161–186. doi: 10.1002/cne.903520202. [DOI] [PubMed] [Google Scholar]
- 33.Goldman-Rakic PS. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 34.Voytko ML. Behav Neurosci. 2002;116:187–197. doi: 10.1037//0735-7044.116.2.187. [DOI] [PubMed] [Google Scholar]
- 35.Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 36.Zuo Y, Lin A, Chang P, Gan WB. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Miller EK. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 38.Sherwin BB. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 39.Maki PM. Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Gibbs RB, Gabor R. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez A, Ehlenberger D, Kelliher K, Einstein M, Henderson SC, Morrison JH, Hof PR, Wearne SL. Methods. 2003;30:94–105. doi: 10.1016/s1046-2023(03)00011-2. [DOI] [PubMed] [Google Scholar]