Abstract
Acute stress impairs memory retrieval and facilitates the induction of long-term depression (LTD) in the hippocampal CA1 region of the adult rodent brain. However, whether such alterations in synaptic plasticity cause the behavioral effects of stress is not known. Here, we report that two selective inhibitors of the induction or expression of stress-enabled, N-methyl-d-aspartate receptor-dependent hippocampal LTD also block spatial memory retrieval impairments caused by acute stress. Additionally, we demonstrate that facilitating the induction of hippocampal LTD in vivo by blockade of glutamate transport mimics the behavioral effects of acute stress by impairing spatial memory retrieval. Thus, the present study demonstrates that hippocampal LTD is both necessary and sufficient to cause acute stress-induced impairment of spatial memory retrieval and provides a new perspective from which to consider the nature of cognitive deficits in disorders whose symptoms are aggravated by stress.
Keywords: glutamate transporter, interference peptide, synaptic plasticity, water maze, corticosterone
Cognitive functions such as learning and memory are greatly affected by stress. Memory retrieval in humans is especially vulnerable to acute psychological stress (1) or cortisol treatment (2), effects caused in part by alterations in medial temporal lobe function (3). In rodents, acute stress or administration of glucocorticoids disrupts the retrieval of hippocampal-dependent spatial memory (4). Furthermore, stress and glucocorticoids have a profound influence on the physiology of the hippocampal CA1 region by inhibiting long-term potentiation (LTP) (5–7) and enabling long-term depression (LTD) (7, 8), the two most well characterized forms of synaptic plasticity and proposed cellular substrates for learning and memory (9, 10). However, it remains to be established whether the alterations in either LTP or LTD caused by stress contribute to the stress-induced impairment of spatial memory retrieval.
Considerable experimental evidence supports the role of hippocampal LTP in spatial memory (11–14), and theoretical accounts of associative memory, based on neural network models, suggest that a balance between LTP and LTD may underlie efficient memory storage (10, 15). By using two recently developed specific inhibitors of LTD (16, 17), the present experiments assess the role of LTD in the spatial memory retrieval deficits induced by acute stress and provide strong evidence for a role of hippocampal LTD in mediating this aspect of acute stress-induced impairment of cognitive function in adult rats.
Results
Blocking the Induction of LTD Prevents the Stress-Induced Impairment of Spatial Memory Retrieval.
It is well accepted that the induction of hippocampal CA1 homosynaptic LTD depends on the N-methyl-d-aspartate subtype of glutamate receptors (NMDARs) (10), which are heteromeric complexes of NR1 subunits and at least one type of NR2 subunit (NR2A–D) (18). Converging evidence supports the hypothesis that the subunit composition of NMDARs may confer distinct roles on the receptors in normal and pathological brain function (18). In particular, several studies using in vitro brain slices prepared from both young and adult rodents provide evidence for a critical role of NR2B-containing NMDAR activation in the induction of hippocampal CA1 LTD (8, 17, 19, 20). However, contradictory results have recently been reported by others (21, 22). Because results both for and against a critical involvement of NR2B-containing receptors were independently obtained from more than one laboratory, it is likely that the subunit requirements for LTP and LTD may be state-dependent phenomena, and these contradictory results may be caused in part by different conditions used in the in vitro studies. Given this controversy, it is important to determine whether the subunit-specific requirements observed in vitro extend to in vivo preparations, where LTD can be studied under more physiologically relevant conditions. In particular, confirmation that specific antagonists for NR2B-containing NMDARs block the induction of LTD without affecting LTP would enable the investigation of the potential role of LTD induction in stress-induced memory impairment. Therefore, we first examined whether the specific NR2B antagonist Ro25-6981 (23) could block stress-enabled hippocampal CA1 LTD without affecting LTP in adult rats in vivo.
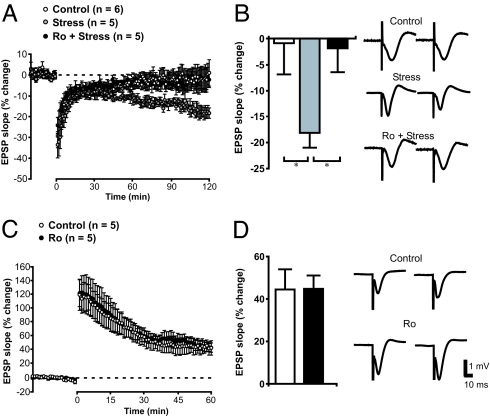
Schaffer collateral stimulation-induced excitatory postsynaptic potentials (EPSPs) were recorded extracellularly in the CA1 stratum radiatum region of hippocampus in anesthetized adult rats. As shown in Fig. 1A, a mild low-frequency stimulation (LFS) protocol failed to produce any notable depression of basal synaptic transmission. However, the same LFS protocol reliably induced LTD in animals subjected to acute stress consisting of a 30-min exposure on an elevated platform (Fig. 1 A and B). Stress treatment did not have a significant effect on basal response magnitude between control animals (slope = −0.18 ± 0.03 mV/ms) and those exposed to the elevated platform (slope = −0.26 ± 0.04 mV/ms). These results are in full agreement with previous reports that acute stress facilitates the induction of hippocampal CA1 LTD in vivo (7). Systemic i.p. application of Ro25-6981 (6 mg/kg of body weight) had no effect on basal EPSPs but did prevent stress-enabled LTD [Fig. 1 A and B; F(2,13) = 3.9, P < 0.05]. The blockade is specific to LTD because the same treatment did not produce significant alteration of LTP elicited by using classical high-frequency stimulation (HFS; Fig. 1C). Thus, similar to results of previous in vitro (8, 17, 19) and in vivo (24, 25) studies, the present experiments strongly suggest that activation of NR2B-containing NMDARs is required for stress-enabled and LFS-induced LTD but not HFS-induced LTP in adult rats in vivo.
Fig. 1.
Acute stress facilitates the induction of NR2B-dependent hippocampal LTD in adult rats in vivo. (A) Evoked-field EPSPs were recorded from the CA1 of anesthetized rats. Although a standard LFS protocol (3 Hz, 900 pulses) failed to induce LTD in intact adult rats (white circles), LTD was readily induced by the same protocol in stressed rats (gray circles). This stress-enabled LTD was completely abolished by the specific NR2B antagonist Ro25-6981 (6 mg/kg, i.p.; black circles). (B) Histogram illustrates the average levels of depression 116–120 min after LTD induction. Representative traces from immediately before tetanus (Left) and 116–120 min after tetanus (Right) are also depicted (*, P < 0.05). (C) Ro25-6981 did not affect LTP induced by HFS. (D) Histogram shows robust potentiation of averaged EPSP (55–60 min after HFS) in control and Ro25-6981-treated rats. Representative traces from immediately before tetanus (Left) and 56–60 min after tetanus (Right) are also depicted. (Scale bars apply to both B and D.)
We then used the NR2B-specific antagonist to investigate the potential contribution of LTD to stress-induced memory impairment in a Morris water maze (MWM) task. Retrieval of spatial memory in the MWM depends on hippocampal NMDARs (26) and is severely impaired by acute stress (4). In the present experiments, rats were trained to remember the location of a hidden platform over eight trials. Twenty-four hours later, their memory for the platform location was tested by using a probe trial with the platform absent from the pool. During the probe trial, trained rats spend significantly longer searching for the platform in the quadrant of the pool where the hidden platform was located during the training phase (Qtest) than in the opposite quadrant (Qopp), demonstrating successful retrieval of spatial memory (Fig. 2A). However, trained rats exposed to the stress induced by a 30-min session on an elevated platform immediately before the probe test performed poorly and spent comparable time searching in the Qtest and Qopp and significantly less time in the Qtest than unstressed rats. Importantly, no impairment in spatial memory retrieval was observed in a visible platform version of the task that does not require the hippocampus [time to find the platform during the recall test 24 h after training: control group 21.3 ± 5.4 s, stress group 27.98 ± 6.6 s, t(14) = −0.79, P = 0.44]. Thus, these results replicate a previous report (4) and confirm that acute stress impairs the retrieval of long-term spatial memory.
Fig. 2.
Systemic injection of Ro25-6981 abolished the impairment of long-term memory retrieval caused by acute stress. (A) Summary of the time spent in the test (Qtest) and opposite quadrants (Qopp) for rats in the control (n = 20), stress (n = 21), and stress + Ro25-6981 groups (n = 16). *, Significant within-group difference between time spent in the Qtest and Qopp (P < 0.05); #, significant between-group difference in time spent in the Qtest (P < 0.05) when the stress group is compared with either of the other groups. (B) Representative swim paths of rats in the control and stress groups during the probe test.
To determine whether this retrieval impairment is the result of the induction of stress-facilitated LTD, we first examined the effect of the specific NR2B antagonist Ro25-6981 on stress-induced memory impairment. Pretreatment with Ro25-6981 (i.p., 6 mg/kg) 30 min before the stress treatment completely abolished the stress-induced impairment of memory retrieval. Analysis of the data with a repeated-measures ANOVA revealed a significant main effect of quadrant [F(1,54) = 40.46, P < 0.001] as well as a significant quadrant by group interaction [F(2,54) = 4.29, P < 0.05]. Post hoc analyses revealed that rats in the stress group spent significantly less time in the test quadrant than rats in the control and Ro25-6981+ stress groups (P < 0.05). Furthermore, treatment of the animals with Ro25-6981 did not affect swimming performance in the MWM (Table 1), indicating that the change in performance was not caused by a change in motor capacity. Additionally, administration of Ro25-6981 to unstressed rats did not affect performance in the MWM (data not shown). Thus, the stress-induced impairment of spatial memory retrieval depends on the activation of NR2B-containing NMDARs.
Table 1.
Ro25-6981 did not affect stress-induced corticosterone (Cort) release or swimming performance of rats
| Parameters | Control | Control + Ro25-6981 | Stress | Stress + Ro25-6981 |
|---|---|---|---|---|
| Blood levels of Cort, μg/dl | 3.65 ± 0.9 (n = 5) | 5.08 ± 0.5 (n = 5) | 35.77 ± 2.1 (n = 6) | 38.70 ± 2.3 (n = 6) |
| Distance swum in probe test, m | 18.59 ± 0.5 (n = 10) | 18.26 ± 0.8 (n = 7) | 17.69 ± 0.6 (n = 13) | 17.96 ± 0.9 (n = 9) |
| Swimming speed, cm/s | 311.30 ± 8.0 (n = 10) | 306.07 ± 14.0 (n = 7) | 291.96 ± 8.3 (n = 13) | 316.29 ± 10.3 (n = 9) |
Note that i.p. injection of Ro25-6981 did not statistically affect any parameter listed in the table.
Impairment in long-term memory retrieval caused by stress is associated with increased levels of the stress hormone corticosterone (4); therefore Ro25-6981 may protect memory retrieval by reducing the stress-induced release of corticosterone. To rule out this possibility, we measured the stress-induced changes in plasma level of corticosterone in the presence and absence of the Ro25-6981 treatment and found that Ro25-6981 did not affect the release of corticosterone caused by elevated platform stress (Table 1).
Blocking the Expression of LTD Prevents the Impairment of Memory Retrieval Caused by Acute Stress.
Because of the recent controversy around the requirement of NR2B-containing receptors in LTD, we felt it essential to employ an LTD-specific inhibitor that is structurally and mechanistically distinct from NR2B receptor antagonists to test the hypothesis that LTD mediates stress-induced impairment of spatial memory retrieval. Although the activation of NMDARs is required for the induction of LTD (10), the expression of LTD appears to involve facilitation of clathrin-dependent endocytosis of postsynaptic α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid subtype of glutamate receptors (AMPARs), through an AMPAR GluR2 subunit-dependent mechanism (27, 28). Through a systematic deletion and carboxyl-terminal truncation, a short stretch of amino acids (869YKEGYNVYG877) in the carboxyl-terminal region of the GluR2 subunit has recently been identified as being essential for the expression of hippocampal CA1 LTD (16). When delivered into postsynaptic neurons, a synthetic peptide containing this sequence of amino acids (GluR23Y) blocks LTD by interfering with the facilitated endocytosis of AMPARs, the last step of LTD expression, without affecting any upstream signaling steps (16, 29). Recent experiments also confirm that administration of the GluR23Y peptide blocks stress-enabled CA1 LTD in young adult rats in vivo (30). Thus, this peptide provides an ideal complement to Ro25-6981 and may be used to confirm that the ameliorative effect of Ro25-6981 on stress-induced memory impairment is caused specifically by blocking the induction of LTD. Accordingly, we also examined the effect of GluR23Y on stress-induced memory disruption.
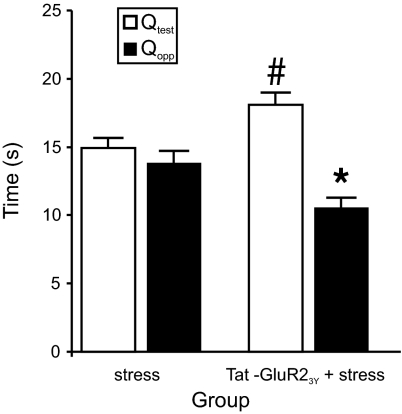
To render the peptide membrane permeable and allow it to be applied systemically to a behaving animal, we fused the peptide to the cell membrane transduction domain of the HIV-1 protein (31) to make a Tat-GluR23Y peptide (YGRKKRRQRRR-869YKEGYNVYG877). This version of the peptide is transported into neurons and exerts its biological effects after systemic administration (29). Separate groups of animals were trained and tested in a manner identical to that used in the NR2B antagonist study except for pretreatment with either the Tat-GluR23Y peptide, saline, or the scrambled control peptide (YGRKKRRQRRR-VYKYGGYNE; 3 μmol/kg; i.p.) 1 h before placement on the elevated platform (because there were no significant differences between the rats treated with saline and scrambled peptide, the groups were combined for statistical analyses). Consistent with an essential role of LTD in stress-induced memory impairment, administration of the Tat-GluR23Y peptide prevented the stress-induced impairment of spatial memory retrieval in the probe test observed in the control group (Fig. 3). Analysis with a repeated-measures ANOVA confirmed these results with a significant main effect of quadrant [F(1,43) = 18.74, P < 0.001] and a significant quadrant by group interaction [F(1,43) = 10.45, P < 0.005]. Post hoc analyses revealed that rats treated with the Tat-GluR23Y peptide before stress spent significantly more time in the test quadrant than rats in the control group (P < 0.05). Thus, these data complement the results found with Ro25-6981 and are consistent with an essential role for expression of LTD in mediating stress-induced deficits in spatial memory retrieval.
Fig. 3.
Systemic injection of Tat-GluR23Y peptide abolished the impairment of long-term memory retrieval due to acute stress. Stressed rats (n = 24) spent a similar amount of time in the test (Qtest) and opposite quadrants (Qopp). However, i.p. injection of Tat-GluR23Y peptide (n = 21) 60 min before stress abolished stress-induced impairment in long-term memory retrieval. *, Significant (P < 0.001) difference between Qtest and Qopp for the Tat-GluR23Y + stress group; #, significant (P < 0.05) difference between stress and Tat-GluR23Y + stress groups.
Facilitating the Induction of Hippocampal CA1 LTD Mimics the Effects of Acute Stress by Disrupting Memory Retrieval.
Finally, we sought to determine whether facilitating the induction of hippocampal CA1 LTD through a means other than acute stress would also impair spatial memory retrieval. A recent study using hippocampal slices provides evidence that the failure to induce consistent LFS-related hippocampal CA1 LTD in adult rats may be caused in part by insufficient activation of NR2B-containing NMDARs located predominantly in extrasynaptic regions (8). Specifically, these authors found that acute stress enables LTD by reducing glutamate transport, which enhances glutamate spillover from the synapse, resulting in activation of extrasynaptic NR2B-containing NMDARs (8). Other studies have reported increased extracellular glutamate levels (32, 33) and glutamate transporter expression (34) in the hippocampus after stress. Given these findings, our final experiment assessed whether blocking glutamate transport with the transporter inhibitor dl-threo-β-benzyloxyaspartate (dl-TBOA) is sufficient to enable CA1 LTD and also disrupt spatial memory retrieval by enabling the activation of extrasynaptically located NR2B-containing NMDARs (Fig. 4A). Consistent with the assertion that LTD is difficult to induce in the adult hippocampus, we observed that a standard protocol (1 Hz, 900 pulses), sufficient to produce LTD in slices prepared from young rats [e.g., 3–4 weeks old (17)], failed to induce LTD in adult rat hippocampal slices at the age (10 weeks) comparable with that used in the present behavioral experiments (Fig. 4B). After inhibition of glutamate transporter activity by 10 μM dl-TBOA (8), bath applied 5 min before LFS to the end of LFS, the same protocol reliably induced LTD in 9 of 10 slices tested [F(2,20) = 3.83, P < 0.05; post hoc, P < 0.05, n = 10], and this LTD was also blocked by NR2B antagonist Ro25-6981 (P = 0.81).
Fig. 4.
Inhibition of hippocampal glutamate transporter activity facilitates the induction of LTD in vitro and in vivo. (A) Schematic depicting the consequences of blocking glutamate transporter (EAAT) activity on the activation of NR2A- and NR2B-containing NMDARs. Under normal conditions, glutamate transport restricts most synaptically released glutamate to the vicinity of the synaptic cleft where it activates AMPARs and NR2A-containing NMDARs. Blocking glutamate transport with dl-TBOA causes glutamate to spill over into the extrasynaptic compartment where it activates NR2B-containing NMDARs and enables the induction of LTD (see B and C). (B) Evoked-field EPSP was recorded from hippocampal slices of adult rats. (Left) LFS protocol (1 Hz, 900 pulses) failed to induce LTD (white circles). LTD was readily induced by the same protocol in dl-TBOA-treated slices (10 μM; gray circles), and this dl-TBOA-enabled LTD was completely abolished by a specific NR2B antagonist Ro25-6981 (3 μM; black circles). Histogram illustrates the average change of the EPSP slope from all groups at 30–35 min after LTD induction (*, P < 0.05). Representative traces from immediately before tetanus (Left) and 30–35 min after tetanus (Right) are also depicted. (C) Evoked-field EPSP was recorded from the CA1 of anesthetized rats in vivo. A standard LFS protocol (3 Hz, 900 pulses) failed to induce LTD in adult rats infused with PBS 20 min before LFS (white circles). LTD was readily induced by the same protocol in rats infused with dl-TBOA (10 nM; gray circles) and was blocked by pretreatment with the specific NR2B antagonist Ro25-6981 30 min before LFS (6 mg/kg, i.p.; black circles). Note that average responses for 2-min intervals are plotted in this panel for clarity. Histogram illustrates the average levels of depression from all groups 116–120 min after LTD induction (*, P < 0.05). Representative traces from immediately before tetanus (Left) and 116–120 min after tetanus (Right) are also depicted.
To test the hypothesis that glutamate transporter blockade would facilitate the induction of LTD in vivo, dl-TBOA was infused (intracerebroventricularly; 10 nM, 5 μl) 20 min before application of the LFS protocol (900 pulses, 3 Hz) to the CA1 region of anesthetized rats. As shown in Fig. 4C, LFS produced LTD in five of six rats that received dl-TBOA [F(2,15) = 6.84, P < 0.01; post hoc, P < 0.01], whereas LTD was not observed in any of the control rats that received a vehicle infusion. Similar to the stress-enabled LTD (Fig. 1A), this facilitation of LTD by glutamate transporter inhibition was also blocked by the NR2B antagonist Ro25-6981 (6 mg/kg; i.p., 30 min before dl-TBOA infusion).
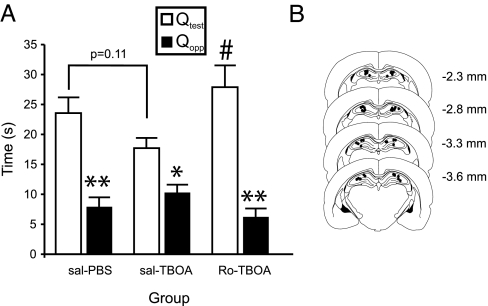
Given that both acute stress and inhibition of glutamate transport with dl-TBOA can facilitate NR2B-containing NMDAR-dependent hippocampal CA1 LTD induction in adult rats, we next investigated whether bilateral infusion of dl-TBOA into the hippocampus could impair retrieval of spatial memory in a manner comparable with that observed in acutely stressed rats. As shown in Fig. 5A, trained rats that received a bilateral intrahippocampal infusion of vehicle (PBS) 20 min before a probe test displayed normal recall and preferentially focused their search in the test quadrant of the water maze. Infusions of dl-TBOA (10 nM, 1 μl) reduced the preference of rats for the test quadrant. Similar to the stress-induced impairment, the disruptive effects of dl-TBOA on memory retrieval were also prevented by Ro25-6981 30 min before the dl-TBOA infusion. Analysis with a repeated-measures ANOVA confirms these results with a significant main effect of quadrant [F(1,33) = 41.94, P < 0.01] and a significant quadrant by group interaction [F(2,33) = 4.02, P < 0.05]. Post hoc analyses revealed that rats treated with Ro25-6981 before dl-TBOA treatment spent significantly more time in the test quadrant than rats treated with saline before dl-TBOA treatment (P < 0.05). Together, these results strongly indicate that the induction of LTD is sufficient to induce an impairment of spatial memory retrieval and likely to be a critical step by which acute stress causes spatial memory impairment.
Fig. 5.
Inhibition of hippocampal glutamate transporter activity disrupts spatial memory retrieval in a NR2B receptor-dependent manner. (A) Summary of the time spent in the test (Qtest) and opposite quadrants (Qopp) for rats in the saline/PBS (n = 8), saline/dl-TBOA (n = 18), and Ro25-6981/dl-TBOA groups (n = 10). The saline and Ro25-6981 injections were given i.p., whereas the PBS and dl-TBOA injections were given intrahippocampally. *, Significant within-group difference between time spent in the Qtest and Qopp (P < 0.05); **, significant within-group difference between time spent in the Qtest and Qopp (P < 0.01); #, significant between-group difference in time spent in the Qtest for the saline/dl-TBOA and Ro25-6981/dl-TBOA groups (P < 0.05). (B) Representative locations of the infusion sites for the rats in the dl-TBOA experiments. Plates were adapted from Paxinos and Watson (47), and their distances posterior from bregma are indicated in millimeters.
Discussion
The present report provides compelling evidence for the role of hippocampal CA1 LTD in stress-induced memory retrieval impairment, specifically related to hippocampal-based spatial learning and memory. As demonstrated previously and convincingly replicated here, acute stress potently facilitates LFS-induced hippocampal CA1 LTD in anesthetized (ref. 35; Fig. 1) and freely moving rats (7) and disrupts the retrieval of spatial memory (ref. 4; Fig. 2). By blocking the induction and expression of hippocampal CA1 LTD with two inhibitors that differ by structure and mechanism of action, we demonstrate an essential requirement for LTD in stress-induced impairment of memory retrieval.
Recent evidence suggests that stress-enabled LTD may result from hippocampal glucocorticoid receptor activation (35, 36), which leads to a reduction in glutamate uptake (8). By using bilateral intrahippocampal infusion of the glutamate transporter inhibitor dl-TBOA, we also demonstrate that facilitation of hippocampal LTD induction is sufficient to impair spatial memory retrieval in a manner similar to that induced by acute stress. These data strongly advance the notion that NR2B-dependent hippocampal LTD is not only necessary but also sufficient to mediate acute stress-induced spatial memory impairment. Data from the present experiments (Figs. 1 and 4) and others (8, 37) support the assertion that inhibition of glutamate uptake produces spillover activation of extrasynaptic NR2B-containing NMDARs, thereby enabling LFS to induce LTD. Recently, it has also been shown that corticosterone, acting through mineralocorticoid receptors, up-regulates glutamate release (38), thereby providing a second mechanism whereby stress could increase glutamate levels in close proximity to glutamatergic synapses and enable LTD by activation of NR2B-containing NMDARs (Fig. 6).
Fig. 6.
Schematic describing the hypothetical mechanisms by which acute stress enables induction of LTD in the hippocampus, thereby causing impaired spatial memory retrieval and the steps at which experimental treatments interfere with this process. The treatments used for experimental induction and inhibition of LTD are indicated in green and red, respectively. Cort, corticosterone.
Determining the neural substrates that contribute to stress-enabled LTD in the hippocampus is also critically important. One likely candidate is the basolateral nucleus of the amygdala (BLA) because substantial evidence shows that BLA lesions can block the impairment of memory and inhibition of hippocampal LTP observed after stress (39). Furthermore, stimulation of the amygdala alters hippocampal synaptic plasticity in a manner similar to stress (40). Thus, it is possible that amygdalar efferents to the hippocampus, when activated by stress, alter hippocampal synaptic plasticity and subsequently impair memory retrieval (15, 41).
How LTD production mediates the acute stress-induced impairment of spatial memory retrieval remains unclear. From a theoretical perspective, memory retrieval (recall) may be a cellular process in which synapses that were potentiated during spatial learning in the MWM are specifically reactivated. Hippocampal CA1 LTD can be produced in an input-specific manner (10); therefore, a plausible conjecture is that acute stress could preferentially produce LTD at only those synapses potentiated during original learning episode, thereby preventing their reactivation during the recall task. Thus, the depressive effects of stress on the hippocampal network during retrieval of specific memories may be restricted to those synapses involved in memory acquisition. Alternatively, acute stress may “reset” the hippocampal network by enabling LTD at all synapses within the network, regardless of whether or not they were potentiated during the original learning episode (15). Accordingly, memory recall would be disrupted by a failure to reactivate a specific subset of synapses potentiated during the original learning episode. One consequence of resetting the network through stress-enabled LTD may be improved performance during subsequent acquisition of new learning. Such a mechanism would be highly adaptive in challenging or stressful environments because it would facilitate learning about potentially dangerous stimuli (15, 42).
Stress-enabled LTD may also compromise memory recall by affecting the balance between LTP and LTD within the hippocampal network. Notably, acute stress impairs HFS-induced hippocampal LTP (5–7) in addition to facilitating induction of LTD. These effects may represent a rightward shift in the Bienenstock–Cooper–Munro curve, a theoretical concept describing the relation between potentiation and depression of synaptic weights within a neural network (10, 43). It is noteworthy that antagonism of NR2B-containing NMDARs with pretreatment of Ro25-6981 not only blocked stress-enabled LTD but also prevented the stress-induced impairment of HFS-induced LTP in the CA1 region (24). Given the previous conjecture that a fine balance between LTP and LTD may be necessary for optimal memory storage, it is possible that normalization of hippocampal LTP after the blockade of stress-enabled LTD also plays a critical role in preventing stress-induced disruption of memory recall. However, determination of the exact role of LTP in stress-induced memory impairment awaits further studies with inhibitors that specifically prevent either the induction and/or expression of LTP.
In summary, the present work provides strong evidence for an essential role of hippocampal LTD in the impairment of spatial memory retrieval induced by acute stress. As depicted in Fig. 6, stress causes the release of corticosterone, which then increases glutamate concentration in the synaptic cleft through increased glutamate release (38) and/or decreased glutamate transport (8) in the hippocampus. The increase in glutamate concentration enables the induction of LTD through spillover activation of extrasynaptically localized NR2B-containing NMDARs (Figs. 1 and 4) and the expression of LTD by facilitating the endocytosis of postsynaptic AMPARs, thereby leading to the impairment of spatial memory retrieval. Stress is a major risk factor affecting hippocampal function in numerous brain disorders including posttraumatic stress disorder and depression (44–46); therefore, an improved understanding of how LTD is facilitated by stress could generate therapeutic targets for these brain diseases.
Materials and Methods
For additional procedures, see supporting information (SI) Methods.
Subjects.
Adult male Sprague–Dawley rats (>300 g; University of British Columbia Animal Care Centre) were pair-housed in plastic cages in a temperature-controlled (21°C) colony room on a 12/12 h light/dark cycle. Food and water were available ad libitum. All experiments were performed in accordance with the Canadian Council on Animal Care and were approved by the University of British Columbia Animal Care Committee.
MWM.
Retrieval of long-term spatial memory was assessed by using an MWM as described (4). Briefly, rats were trained in a fiberglass pool over eight trials to find a hidden platform. During the retrieval phase, rats were returned to the pool from a novel drop point with the hidden platform absent for 60 s. All trials were recorded with a video camera and analyzed by using an EthoVision tracking system (Noldus, Leesburg, VA).
Stressor.
Rats were stressed two at a time on separate elevated Plexiglas platforms (1 m tall, 21 × 21 cm) in a brightly lit room for 30 min immediately before the probe trial. Rats consistently urinated and/or defecated while on the platform and had elevated blood corticosterone levels immediately after stress treatment (Table 1). Ro25-6981 (6 mg/kg) or vehicle (0.9% saline) was administered i.p. 30 min before the stress treatment. Injection of peptides was performed 1 h before the stress treatment.
In Vitro Slice Preparation and Electrophysiological Recording.
Coronal brain slices (400-μm thickness) containing hippocampus were cut in ice-cold artificial cerebrospinal fluid (ACSF). Freshly cut slices were placed in an incubating chamber with carbogenated ACSF and recovered at 34°C for 1.5 h. Slices were then maintained at room temperature before recording. For electrophysiological recordings, slices were transferred to a recording chamber perfused continuously by carbogenated ACSF containing 10 μM bicuculline methiodide to block GABAA receptor-mediated inhibitory synaptic currents. EPSP responses were evoked by stimulating the Schaffer collateral-commissural pathway by a constant current pulse (0.08 ms) delivered through a tungsten bipolar electrode (FHC, Bowdoin, ME). Synaptic responses were evoked at 0.05 Hz except during the induction of LTD. Field EPSPs were recorded by placing a glass pipette filled with ACSF in the stratum radiatum of the CA1 region at least 60–80 μm away from the cell body layer. After obtaining a stable baseline for 10 min, LTD was induced by 900 pulses at 1 Hz. Drugs (dl-TBOA and Ro25-6981) were present in ACSF 5 min before and during LFS stimulation.
In Vivo Electrophysiology.
EPSPs from the CA1 region of the hippocampus were recorded by using described techniques (25). Rats were anesthetized and placed in a stereotaxic frame. The scalp was opened and trephine holes were drilled for the recording and stimulating electrodes, which were then lowered into the CA1 region. Pyramidal cell responses to Schaffer collateral stimulation were recorded, and final depths of the electrodes were determined when a maximal CA1 EPSP could be obtained with minimal stimulation. For all testing, EPSPs were adjusted to ≈60% of the maximal response size. Baseline responses were obtained by applying single pulses of stimulation at 0.067 Hz. LTP was elicited by HFS consisting of four trains of 50 pulses delivered at 100 Hz (15-s intertrain interval). LTD was elicited with a low-frequency tetanus (3 Hz, 300 s). After the tetanus, stimulation was returned to baseline rates, and decay was followed. When necessary, Ro25-6981 was injected (i.p.) 30 min before delivery of the tetanus (Fig. 1 A and B). In some animals (Fig. 4B), either vehicle (0.1 M PBS) or dl-TBOA (10 nM) was injected into the lateral ventricle. At 20 min after the infusion, the low-frequency tetanus was applied. When necessary, Ro25-6981 was applied (i.p.) 10 min before the dl-TBOA infusion.
dl-TBOA Microinfusion Experiments.
Rats were implanted with two 22-gauge stainless steel cannulae above the dorsal hippocampus and allowed to recover for at least 10 days. The rats were extensively habituated to one of two Plexiglas infusion boxes. Water maze testing procedures were identical to those above except that 20 min before the probe test, the rats had needles inserted into their dorsal hippocampi and either vehicle (0.1 M PBS) or dl-TBOA (10 nM) was infused (0.5 μl/min for 2 min). In some cases, Ro25-6981 (6 mg/kg) or saline (0.9%) was administered (i.p.) 30 min before the initiation of the dl-TBOA infusion procedure. After testing, the rats were perfused, and the injection sites were determined with the assistance of a rat brain atlas (47).
Statistical Analysis.
Water maze.
All data are presented as mean ± SEM. The time spent in the test and opposite quadrants during the probe test in each experiment (Ro25-6981, Tat-GluR23Y peptide, and dl-TBOA) was analyzed with a repeated-measures ANOVA. Post hoc analyses were conducted by using Fisher's test for between-subjects and paired t tests for within-subjects comparisons.
Electrophysiological experiments.
All data are expressed as the average percentage change from baseline ± SEM and were analyzed by one-way ANOVA followed by post hoc tests (Fisher's test) where appropriate.
Supplementary Material
Acknowledgments
This work was supported by Canadian Institutes of Health Research (CIHR) Grant M0P-38090 and NeuroScience Canada. Y.T.W. is a Howard Hughes Medical Institute International Scholar, CIHR Investigator, Michael Smith Foundation for Health Research (MSFHR) Senior Scholar, and Heart and Stroke Foundation of British Columbia and the Yukon Chair in Stroke Research. T.P.W. and J.G.H. were supported by fellowships from CIHR and MSFHR. Y.T.W. received support from CIHR. Y.T.W. and A.G.P. received support from the Neuroscience Canada Brain Repair Program.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AMPAR
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor
- dl-TBOA
dl-threo-β-benzyloxyaspartate
- EPSP
excitatory postsynaptic potential
- HFS
high-frequency stimulation
- LFS
low-frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- MWM
Morris water maze
- NMDAR
N-methyl-d-aspartate receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702308104/DC1.
References
- 1.Kuhlmann S, Piel M, Wolf OT. J Neurosci. 2005;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 3.de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C. Eur J Neurosci. 2003;17:1296–1302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- 4.de Quervain DJ, Roozendaal B, McGaugh JL. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 5.Diamond DM, Fleshner M, Rose GM. Behav Brain Res. 1994;62:1–9. doi: 10.1016/0166-4328(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 6.Shors TJ, Seib TB, Levine S, Thompson RF. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Anwyl R, Rowan MJ. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 8.Yang CH, Huang CC, Hsu KS. J Neurosci. 2005;25:4288–4293. doi: 10.1523/JNEUROSCI.0406-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bliss TV, Collingridge GL. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 10.Malenka RC, Bear MF. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 12.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 13.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 14.Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 15.Diamond DM, Park CR, Campbell AM, Woodson JC. Hippocampus. 2005;15:1006–1025. doi: 10.1002/hipo.20107. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadian G, Ju W, Liu L, Wyszynski M, Lee SH, Dunah AW, Taghibiglou C, Wang Y, Lu J, Wong TP, et al. EMBO J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 18.Cull-Candy S, Brickley S, Farrant M. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 19.Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 20.Izumi Y, Auberson YP, Zorumski CF. J Neurosci. 2006;26:7181–7188. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendricson AW, Miao CL, Lippmann MJ, Morrisett RA. J Pharmacol Exp Ther. 2002;301:938–944. doi: 10.1124/jpet.301.3.938. [DOI] [PubMed] [Google Scholar]
- 22.Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Neuropharmacology. 2007;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Mutel V, Buchy D, Klingelschmidt A, Messer J, Bleuel Z, Kemp JA, Richards JG. J Neurochem. 1998;70:2147–2155. doi: 10.1046/j.1471-4159.1998.70052147.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Yang Y, Dong Z, Cao J, Xu L. Neuroreport. 2006;17:1343–1346. doi: 10.1097/01.wnr.0000227994.07799.6c. [DOI] [PubMed] [Google Scholar]
- 25.Fox CJ, Russell KI, Wang YT, Christie BR. Hippocampus. 2006;16:907–915. doi: 10.1002/hipo.20230. [DOI] [PubMed] [Google Scholar]
- 26.Morris RG, Anderson E, Lynch GS, Baudry M. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 27.Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 28.Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 29.Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, Phelps L, Phillips AG, Wang YT. Science. 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 30.Fox CJ, Russell K, Titterness AK, Wang YT, Christie BR. Hippocampus. 2007 doi: 10.1002/hipo.20302. in press. [DOI] [PubMed] [Google Scholar]
- 31.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 32.Lowy MT, Wittenberg L, Yamamoto BK. J Neurochem. 1995;65:268–274. doi: 10.1046/j.1471-4159.1995.65010268.x. [DOI] [PubMed] [Google Scholar]
- 33.Lowy MT, Gault L, Yamamoto BK. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 34.Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, McEwen BS. Proc Natl Acad Sci USA. 2004;101:2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L, Holscher C, Anwyl R, Rowan MJ. Proc Natl Acad Sci USA. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavlides C, Kimura A, Magarinos AM, McEwen BS. Neuroscience. 1995;68:379–385. doi: 10.1016/0306-4522(95)94332-s. [DOI] [PubMed] [Google Scholar]
- 37.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JJ, Koo JW, Lee HJ, Han JS. J Neurosci. 2005;25:1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akirav I, Richter-Levin G. J Neurosci. 1999;19:10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGaugh JL. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 42.Roozendaal B. Neurobiol Learn Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 43.Bienenstock EL, Cooper LN, Munro PW. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holsboer F. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 45.Sapolsky RM. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 46.McEwen BS. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.