Abstract
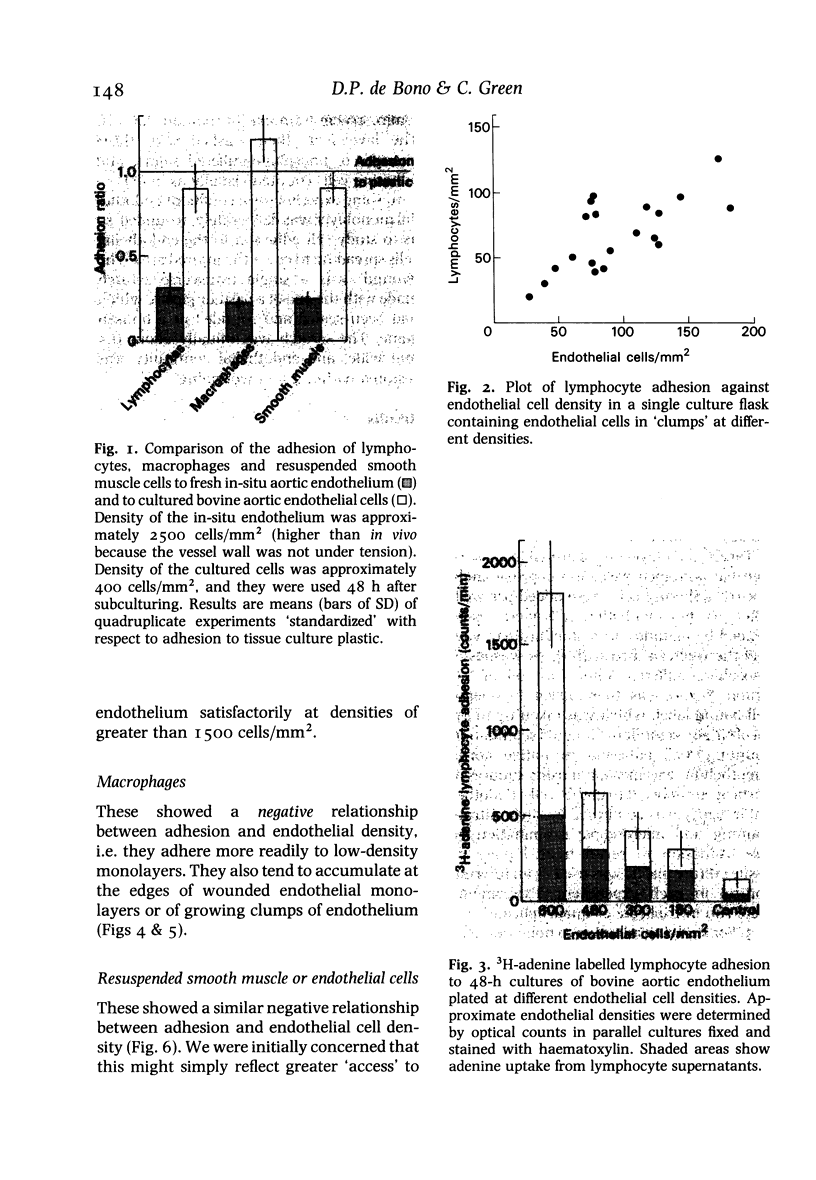
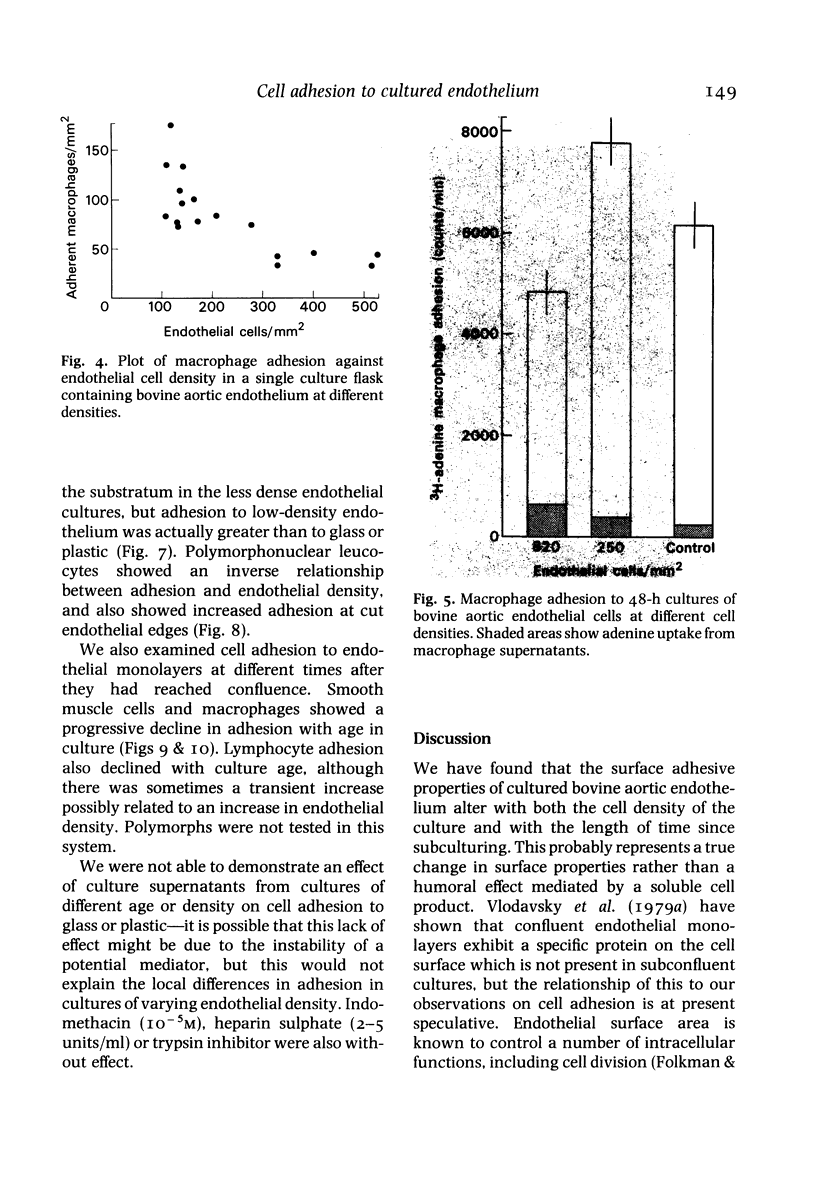
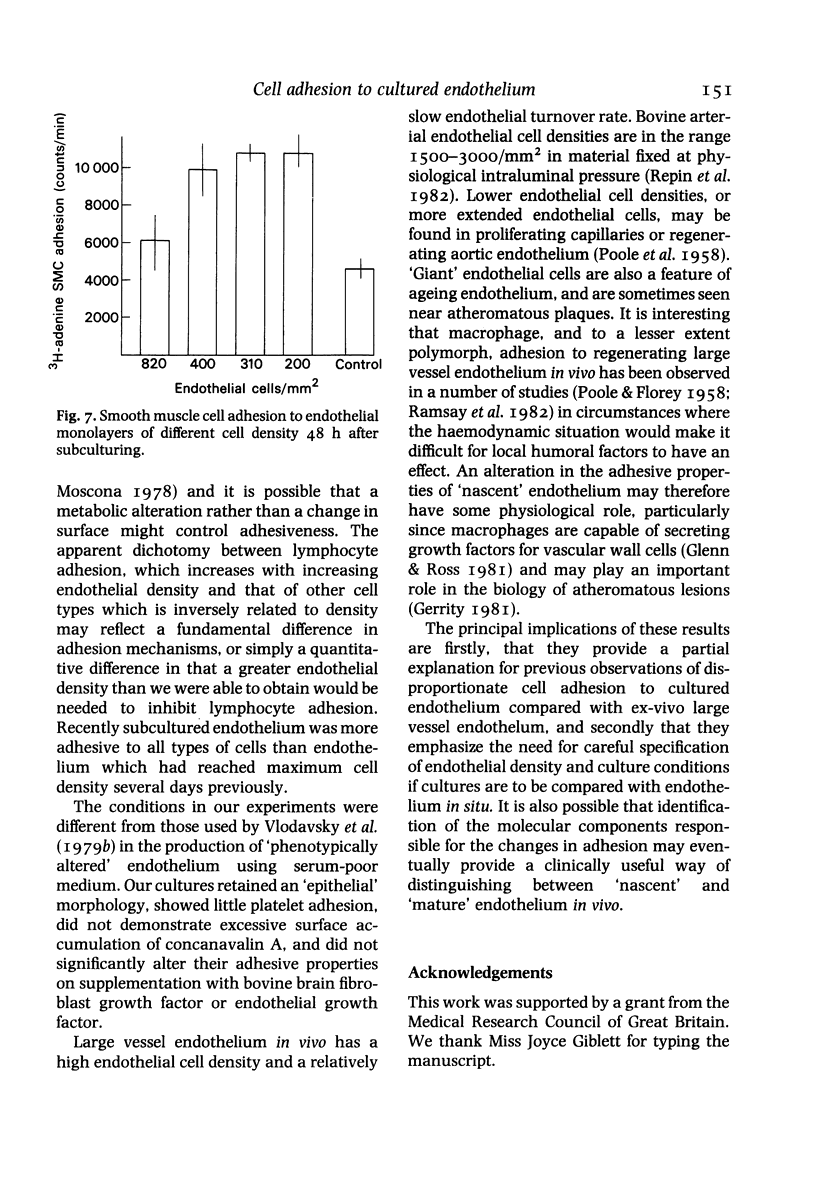
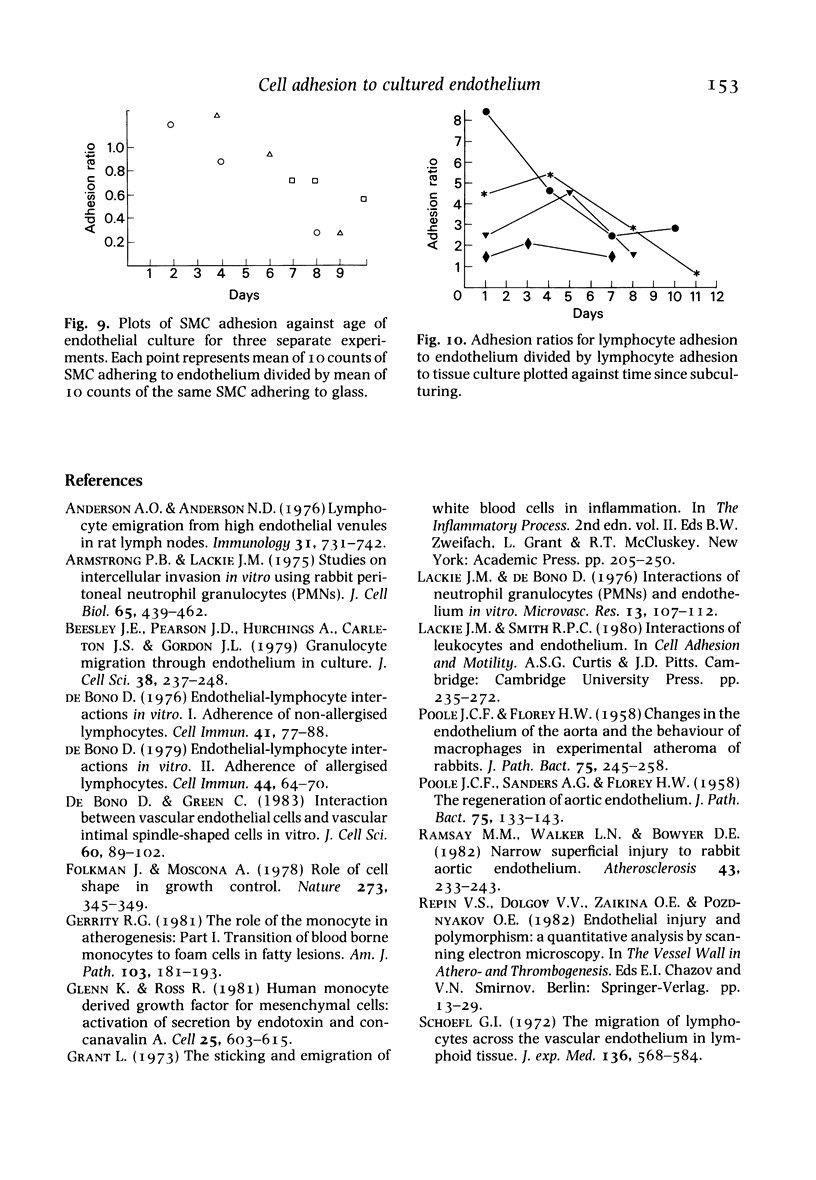
The adhesion of lymphocytes, macrophages and resuspended smooth muscle cells to freshly subcultured bovine aortic endothelial cells is considerably greater than their adhesion to in-situ aortic endothelium when tested in vitro. Experiments with endothelial monolayers of different cell density and maturity suggest that this can be explained, at least in part, by two factors--firstly, an inverse relationship between macrophage, polymorph and smooth muscle cell (but not lymphocyte) adhesion and endothelial cell density, and secondly, an inverse relationship between endothelial adhesiveness and time since a culture became confluent. These observations may help to clarify the relationship between endothelial adhesiveness in vitro and in vivo, and to explain why leucocytes tend to adhere to regenerating arterial endothelium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong P. B., Lackie J. M. Studies of intercellular invasion in vitro using rabbit peritoneal neutrophil granulocytes (PMNS). I. Role of contact inhibition of locomotion. J Cell Biol. 1975 May;65(2):439–462. doi: 10.1083/jcb.65.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn K. C., Ross R. Human monocyte-derived growth factor(s) for mesenchymal cells: activation of secretion by endotoxin and concanavalin A. Cell. 1981 Sep;25(3):603–615. doi: 10.1016/0092-8674(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Lackie J. M., de Bono D. Interactions of neutrophil granulocytes (PMNs) and endothelium in vitro. Microvasc Res. 1977 Jan;13(1):107–112. doi: 10.1016/0026-2862(77)90119-4. [DOI] [PubMed] [Google Scholar]
- POOLE J. C., SANDERS A. G., FLOREY H. W. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958 Jan;75(1):133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- Ramsay M. M., Walker L. N., Bowyer D. E. Narrow superficial injury to rabbit aortic endothelium. The healing process as observed by scanning electron microscopy. Atherosclerosis. 1982 Jun;43(2-3):233–243. doi: 10.1016/0021-9150(82)90025-9. [DOI] [PubMed] [Google Scholar]
- Schoefl G. I. The migration of lymphocytes across the vascular endothelium in lymphoid tissue. A reexamination. J Exp Med. 1972 Sep 1;136(3):568–588. doi: 10.1084/jem.136.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono D., Green C. Interaction between vascular endothelial cells and vascular intimal spindle-shaped cells in vitro. J Cell Sci. 1983 Mar;60:89–102. doi: 10.1242/jcs.60.1.89. [DOI] [PubMed] [Google Scholar]





