Abstract
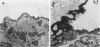
Pneumocystis carinii infection is characterized by the attachment of P. Carinii to host alveolar type I cell and propagation of the organism with corticosteroid administration. We have examined the effects of corticosteroids on the cell surface glycocalyx of the pulmonary alveolus in rats by ultrastructural histochemistry using cationized ferritin, ruthenium red and concanavalin A-horseradish peroxidase techniques. In rats treated for 4 and 6 weeks, the amount of the alveolar cell surface glycocalyx was markedly decreased by all three techniques. The changes were most pronounced at the cell surface of the type I pneumocyte, and this may be important in the pathogenesis of P. carinii pneumonia.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard P. L., Tomkins G. M. Hormone induced modification of the cell surface. Nature. 1969 Oct 25;224(5217):344–345. doi: 10.1038/224344a0. [DOI] [PubMed] [Google Scholar]
- Baumann H., Chu F. F. Altered composition of membrane glycoconjugates in rat hepatoma tissue culture cells after dexamethasone treatment or in vivo growth. Cancer Res. 1979 Sep;39(9):3540–3548. [PubMed] [Google Scholar]
- Baumann H., Gelehrter T. D., Doyle D. Dexamethasone regulates the program of secretory glycoprotein synthesis in hepatoma tissue culture cells. J Cell Biol. 1980 Apr;85(1):1–8. doi: 10.1083/jcb.85.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R. E. Ruthenium red stainable surface layer on lung alveolar cells; electron microscopic interpretation. Stain Technol. 1969 Jul;44(4):173–177. doi: 10.3109/10520296909063346. [DOI] [PubMed] [Google Scholar]
- Chandler D. K., Grabowski M. W., Barile M. F. Mycoplasma pneumoniae attachment: competitive inhibition by mycoplasmal binding component and by sialic acid-containing glycoconjugates. Infect Immun. 1982 Nov;38(2):598–603. doi: 10.1128/iai.38.2.598-603.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C., Balow J. E. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976 Mar;84(3):304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Parsons C. L., Stauffer C., Schmidt J. D. Bladder-surface glycosaminoglycans: an efficient mechanism of environmental adaptation. Science. 1980 May 9;208(4444):605–607. doi: 10.1126/science.6154316. [DOI] [PubMed] [Google Scholar]
- Simionescu D., Simionescu M. Differentiated distribution of the cell surface charge on the alveolar-capillary unit. Characteristic paucity of anionic sites on the air-blood barrier. Microvasc Res. 1983 Jan;25(1):85–100. doi: 10.1016/0026-2862(83)90045-6. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Baron D. A., Sato A., Schulte B. A. Variability of cell surface glycoconjugates--relation to differences in cell function. J Histochem Cytochem. 1981 Sep;29(9):994–1002. doi: 10.1177/29.9.6169762. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K. Experimental Pneumocystis carinii pneumonia in different strains of cortisonized mice. Infect Immun. 1979 Jun;24(3):939–947. doi: 10.1128/iai.24.3.939-947.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K., Rutledge M. E., Milder J. E. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980 Mar;27(3):928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Attachment of Pneumocystis carinii to type I alveolar cells studied by freeze-fracture electron microscopy. Infect Immun. 1983 May;40(2):812–815. doi: 10.1128/iai.40.2.812-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Interaction of Pneumocystis carinii with host lungs: an ultrastructural study. Infect Immun. 1980 Aug;29(2):692–703. doi: 10.1128/iai.29.2.692-703.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Mechanism of pulmonary alveolar injury in experimental Pneumocystis carinii pneumonia in the rat. Br J Exp Pathol. 1981 Aug;62(4):339–346. [PMC free article] [PubMed] [Google Scholar]