Abstract
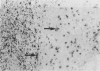
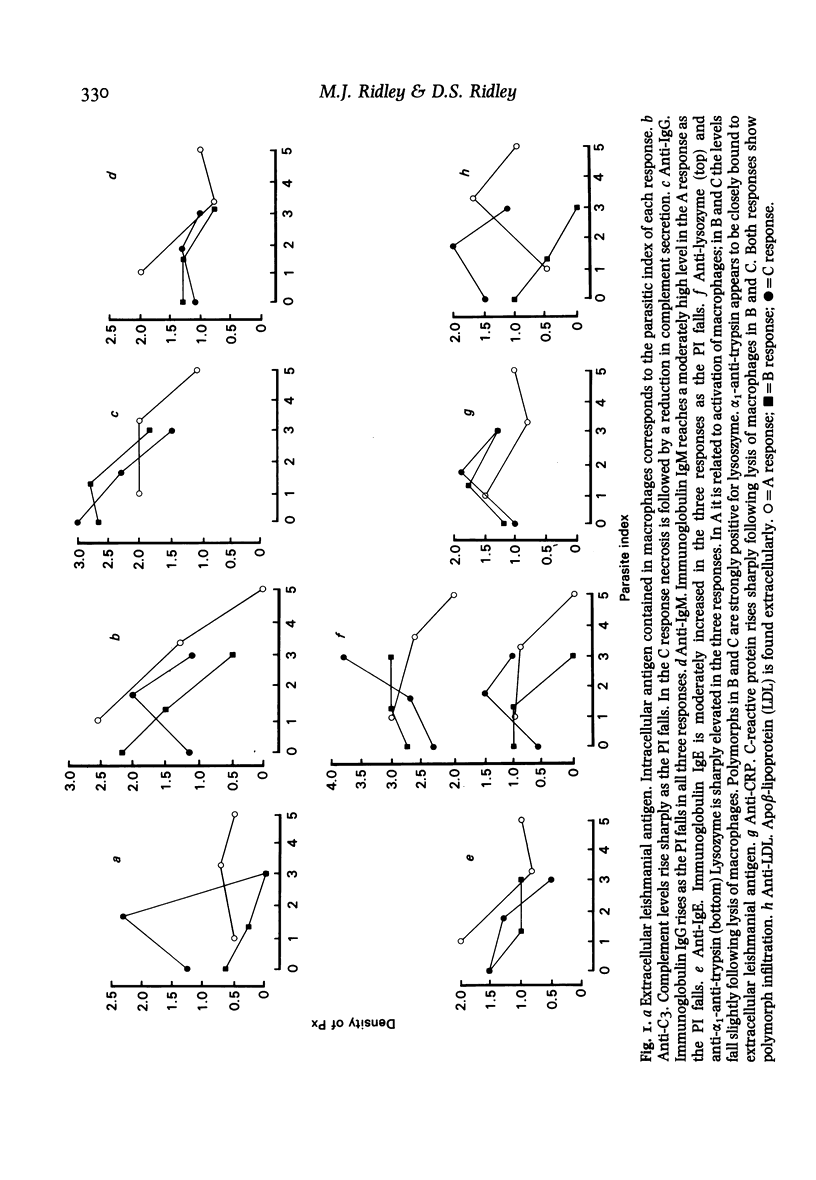
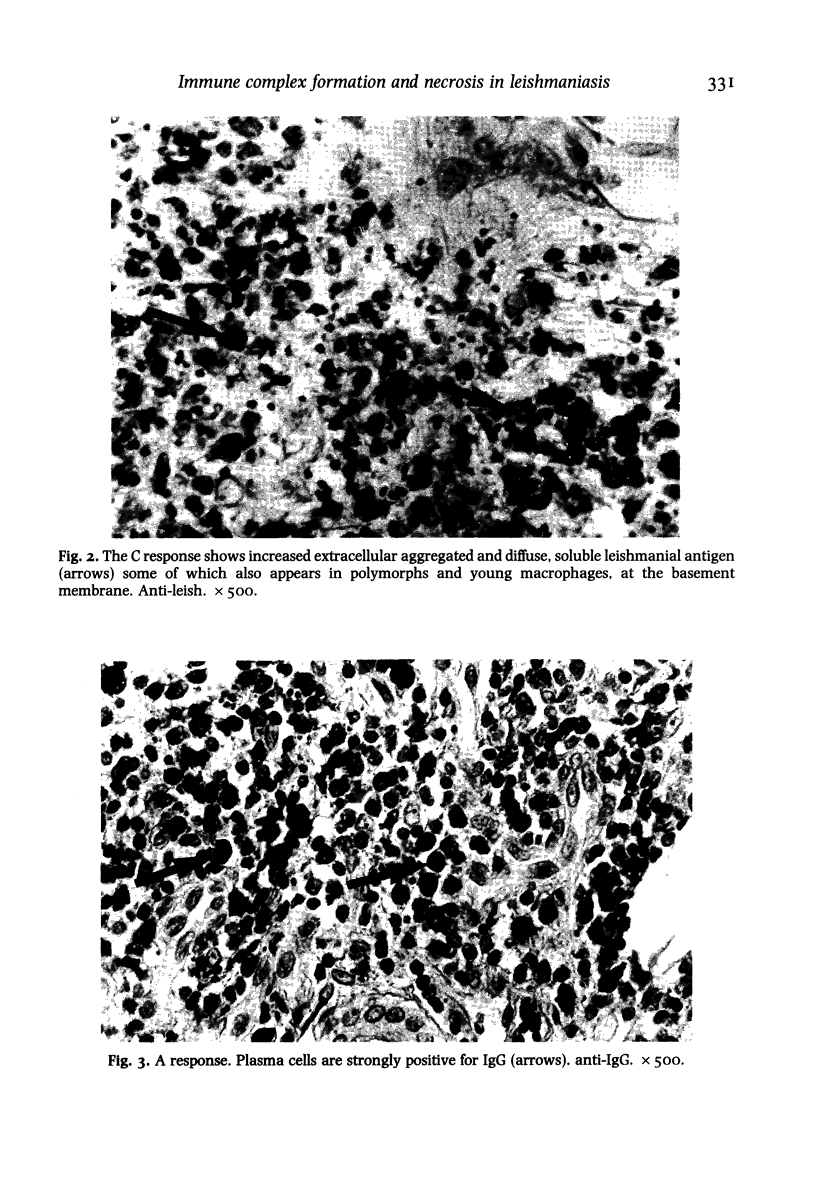
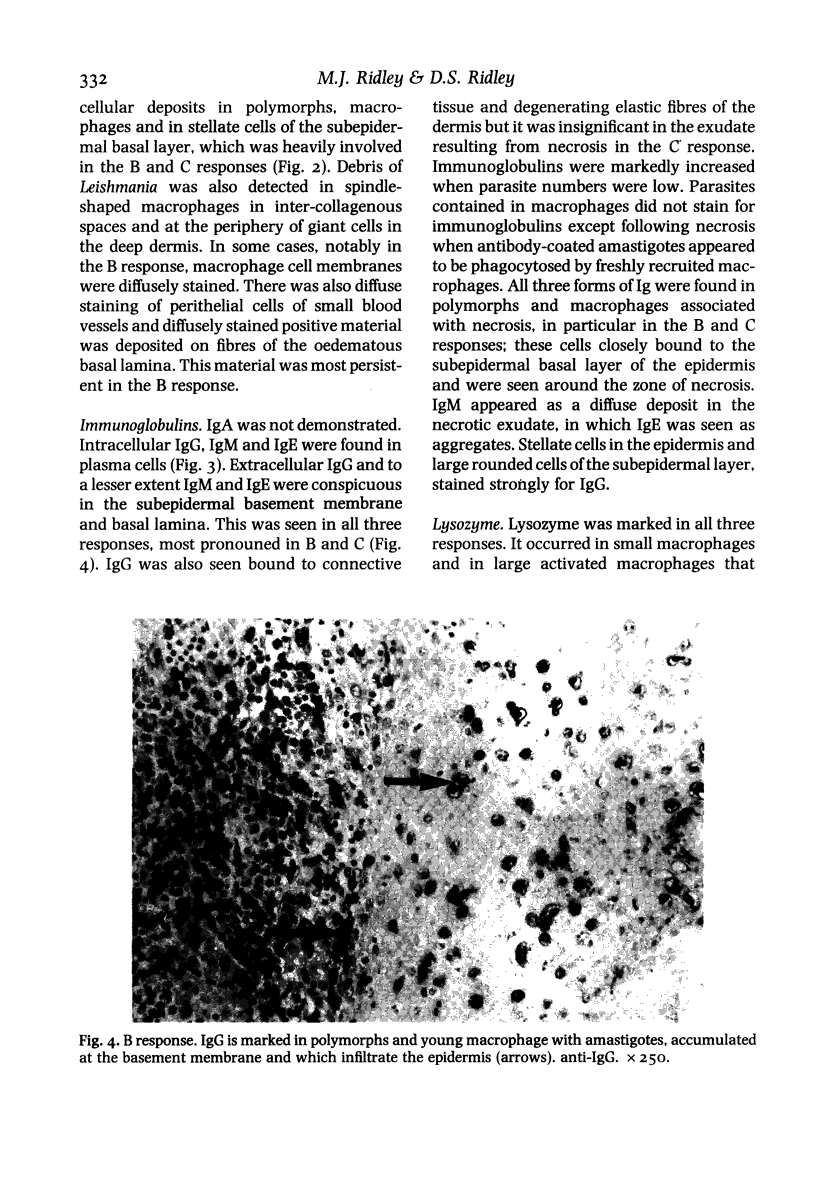
Twenty biopsies of lesions of cutaneous leishmaniasis were classified according to the mechanism of parasite elimination, on the basis of macrophage activation (five cases) or macrophage lysis (15 cases). The immunoperoxidase technique was used to demonstrate free Leishmania antigen, immunoglobulins, complement, lysozyme, C-reactive protein, beta-lipoprotein, alpha 1-antitrypsin, alpha 2-macroglobulin, plasminogen and factor VIII, which were quantitated and comparatively assessed. The fall in the parasite load during the course of the infection was associated with rising levels of IgG, IgM and IgE, and of the complement components of the classical pathway. Macrophage lysis supervened when there was an approximate equivalence of antigen and antibody, and was associated with the deposition of immune complex components. Lysis of the acute focal type (C response) was accompanied by a massive liberation of free Leishmania antigen, followed by a fall indicative of parasite elimination. The lysis of small numbers of macrophages scattered diffusely in the lesion, which was slow to reach completion (B response), was less effective and immunologically closer to the non-lytic (A) response. A terminal fall of the immunological factors other than the globulins, suggestive of resolution, was observed mainly in the C response. Lymphocytes may be important in macrophage activation associated with the macrophage A response and in the later stage of the B and C responses. However immunologically induced host-cell lysis is more important than macrophage activation for the elimination of Leishmania in the acute stage of most skin lesions. It is associated with, and may be caused by, the formation in situ of immune complexes of Leishmania antigen and antibody at an appropriate ratio.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckers H. J., van der Hoeven J. S. Effect of microbial interaction on the colonization rate of Actinomyces viscosus or Streptococcus mutans in the dental plaque of rats. Infect Immun. 1982 Oct;38(1):8–13. doi: 10.1128/iai.38.1.8-13.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D., Bray R. S., Wolstencroft R. A., Dumonde D. C. Immunity in cutaneous leishmaniasis of the guinea-pig. Clin Exp Immunol. 1970 Sep;7(3):301–341. [PMC free article] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Joseph M., Rousseaux R., Capron M., Bazin H. Interaction between IgE complexes and macrophages in the rat: a new mechanism of macrophage activation. Eur J Immunol. 1977 May;7(5):315–322. doi: 10.1002/eji.1830070515. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Leishmania donovani. Hamster macrophage interactions in vitro: cell entry, intracellular survival, and multiplication of amastigotes. J Exp Med. 1978 Feb 1;147(2):515–530. doi: 10.1084/jem.147.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth J. A., Williams D. G., Sherington E., Lachmann P. J., Peters D. K. Metabolic studies of the third component of complement and the glycine-rich beta glycoprotein in patients with hypocomplementemia. J Clin Invest. 1974 Jun;53(6):1578–1587. doi: 10.1172/JCI107708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. C., Gregory J. Effects of fixation and processing on immunohistochemical demonstration of immunoglobulin in paraffin sections of tonsil and bone marrow. J Clin Pathol. 1980 Nov;33(11):1047–1057. doi: 10.1136/jcp.33.11.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. M. Antibody-induced modulation of Leishmania donovani surface membrane antigens. J Immunol. 1976 Dec;117(6):2081–2091. [PubMed] [Google Scholar]
- Farah F. S., Samra S. A., Nuwayri-Salti N. The role of the macrophage in cutaneous leishmaniasis. Immunology. 1975 Oct;29(4):755–764. [PMC free article] [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Lynch N. R., Yarzábal L., Verde O., Avila J. L., Monzon H., Convit J. Delayed-type hypersensitivity and immunoglobulin E in American cutaneous leishmaniasis. Infect Immun. 1982 Dec;38(3):877–881. doi: 10.1128/iai.38.3.877-881.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriearty P. L., Grimaldi G., Jr, Galvão-Castro B., de Oliveira Neto M. P., Marzochi M. C. Intralesional plasma cells and serological responses in human cutaneous leishmaniasis. Clin Exp Immunol. 1982 Jan;47(1):59–64. [PMC free article] [PubMed] [Google Scholar]
- Petersen E. A., Neva F. A., Oster C. N., Bogaert Diaz H. Specific inhibition of lymphocyte-proliferation responses by adherent suppressor cells in diffuse cutaneous leishmaniasis. N Engl J Med. 1982 Feb 18;306(7):387–392. doi: 10.1056/NEJM198202183060702. [DOI] [PubMed] [Google Scholar]
- Radwanski Z. K., Bryceson A. D., Preston P. M., Dumonde D. C. Immunoflorescence studies of Leishmania enriettii infection in the guinea pig. Trans R Soc Trop Med Hyg. 1974;68(2):124–132. doi: 10.1016/0035-9203(74)90185-0. [DOI] [PubMed] [Google Scholar]
- Ridley D. S. A histological classification of cutaneous leishmaniasis and its geographical expression. Trans R Soc Trop Med Hyg. 1980;74(4):515–521. doi: 10.1016/0035-9203(80)90069-3. [DOI] [PubMed] [Google Scholar]
- Ridley M. J., Ridley D. S. The immunopathology of erythema nodosum leprosum: the role of extravascular complexes. Lepr Rev. 1983 Jun;54(2):95–107. doi: 10.5935/0305-7518.19830015. [DOI] [PubMed] [Google Scholar]
- Ridley M. J., Ridley D. S., Willoughby D. A. Extravascular immune complexes in experimental mycobacterial BCG granulomas. J Pathol. 1983 Dec;141(4):469–482. doi: 10.1002/path.1711410405. [DOI] [PubMed] [Google Scholar]