Abstract
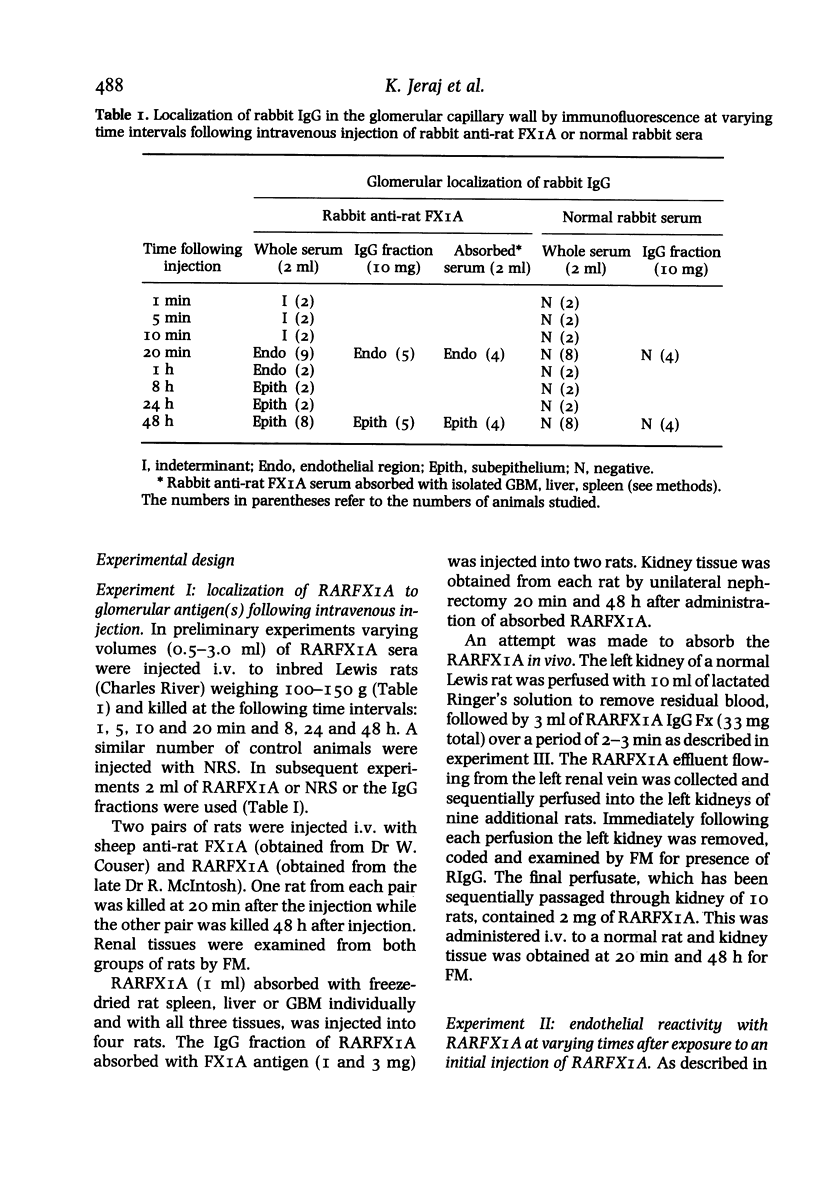
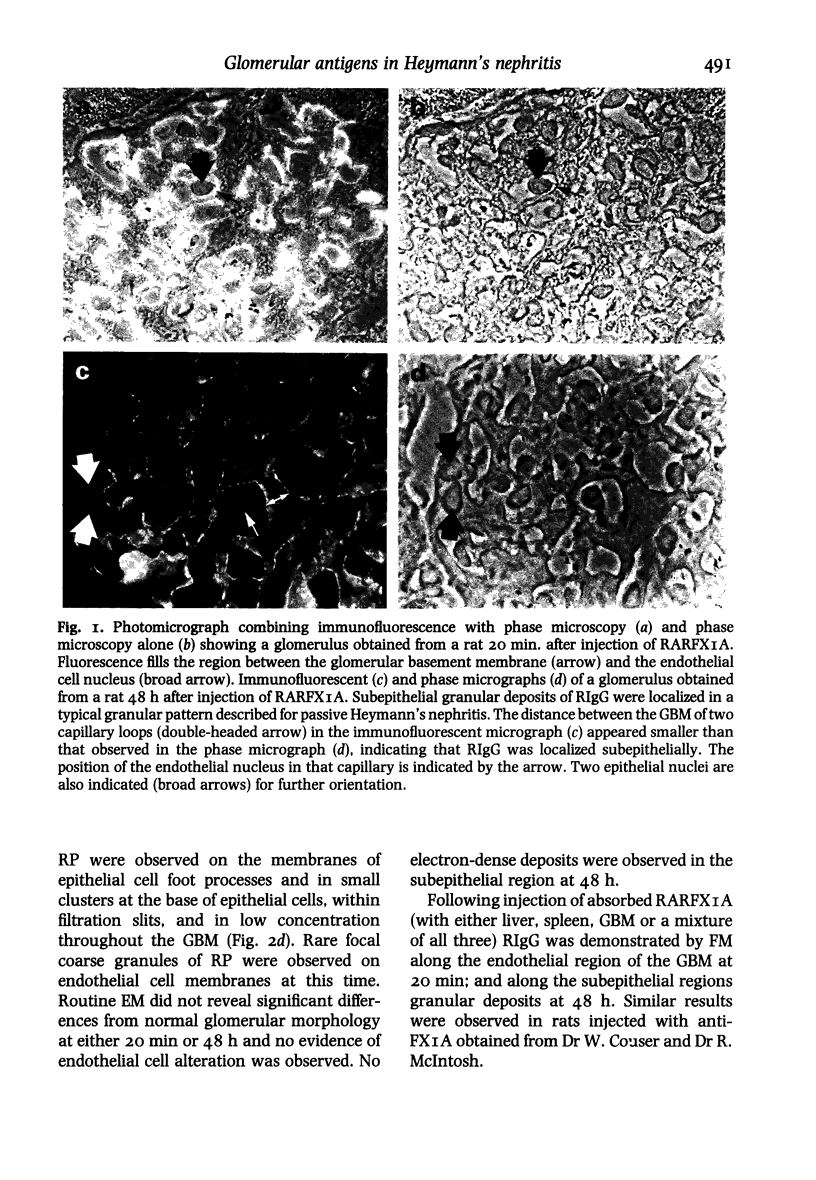
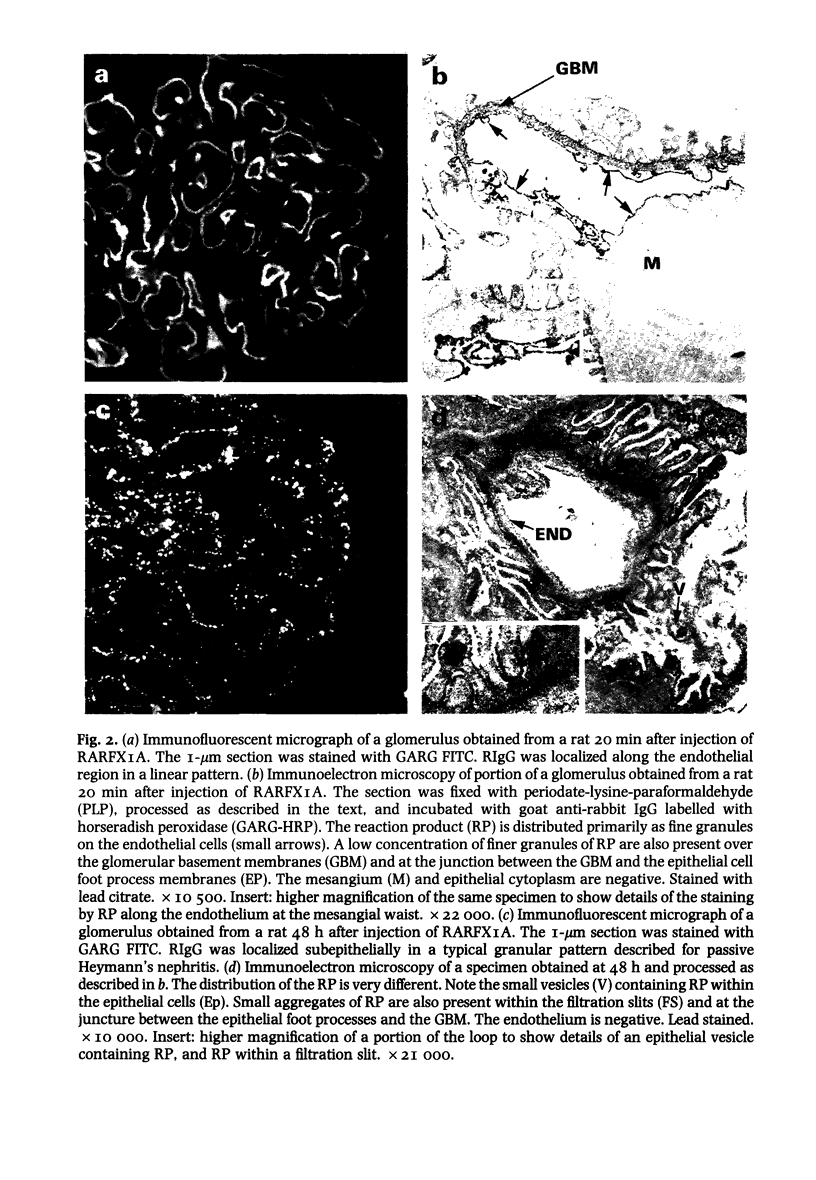
Within 20 min after i.v. injection or unilateral renal perfusion of rabbit anti-rat proximal tubular brush border antigens (RARFAXIA) into rats, fluorescence microscopy (FM) demonstrated rabbit IgG (RIgG) in a linear fashion along the endothelial region of the glomerular capillary walls. This finding was confirmed by immuno-electron microscopy (IEM) which revealed the presence of reaction product on the plasma membranes of the endothelial cells. Between 8 h and 26 days following i.v. injection of RARFXIA, granular subepithelial deposits of RIgG were demonstrated by FM and IEM, and the endothelial localization seen at earlier time periods was no longer present. In the later time periods after loss of RIgG from the endothelial region, a second injection of RARFXIA did not result in binding of IgG to this site suggesting loss of the antigen or impairment in antigen-antibody binding affinity. Evidence for depletion of endothelial binding antibody from the circulation was derived from passive transfer experiments, in which sera were harvested from rats either 20 min or 48 h following i.v. injection of RARFXIA-I125. When equivalent doses of these sera were perfused into kidneys of normal rats, minimal glomerular binding was demonstrated with sera obtained at 20 min, but no binding to the capillary wall was observed with sera obtained at 48 h. These observations demonstrate that immediately after the induction of passive Heymann's nephritis (PHN) with the complex polyclonal antibody to FXIA, an antigen-antibody reaction occurs along the endothelial region of the glomerular capillary and that later in the course of the disease in vivo, antibody binding to this site is abrogated. The relationship of this early event to the ultimate development of subepithelial deposits is unknown. This reaction may be a source of immune complexes which migrate through the glomerular basement membrane (GBM) or the early binding of the antibody to an endothelial antigen(s) may result in altered permeability of the glomerular capillary allowing other antibodies to find their putative antigen(s).
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Druet P., Bariéty J., Laliberté F., Bellon B., Belair M. F., Paing M. Distribution of heterologous antiperoxidase antibodies and their fragments in the superficial renal cortex of normal Wistar-Munich rat: an ultrastructural study. Lab Invest. 1978 Dec;39(6):623–631. [PubMed] [Google Scholar]
- Germuth F. G., Jr, Rodriguez E., Lorelle C. A., Trump E. I., Milano L. L., Wise O. Passive immune complex glomerulonephritis in mice: models for various lesions found in human disease. II. Low avidity complexes and diffuse proliferative glomerulonephritis with subepithelial deposits. Lab Invest. 1979 Oct;41(4):366–371. [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A., Niwa Y., Shigematsu H., Taniguchi M., Tada T. Studies on passive serum sickness. II. Factors determining the localization of antigen-antibody complexes in the murine renal glomerulus. Lab Invest. 1978 Mar;38(3):253–262. [PubMed] [Google Scholar]
- Lee S., Vernier R. L. Immunoelectron microscopy of the glomerular mesangial uptake and transport of aggregated human albumin in the mouse. Lab Invest. 1980 Jan;42(1):44–58. [PubMed] [Google Scholar]
- Nevins T. E., Michael A. F. Isolation of anionic sialoproteins from the rat glomerulus. Kidney Int. 1981 Apr;19(4):553–563. doi: 10.1038/ki.1981.52. [DOI] [PubMed] [Google Scholar]
- Patton S., Bogus E. R., Stemberger B. H., Trams E. G. Antiserum to the milk fat globule membrane. Preparation and capacity to suppress milk secretion. Biochim Biophys Acta. 1980 Apr 10;597(2):216–233. doi: 10.1016/0005-2736(80)90100-5. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Hein S. J., Karnovsky M. J. Glomerular permeability to proteins. Effects of hemodynamic factors on the distribution of endogenous immunoglobulin G and exogenous catalase in the rat glomerulus. Lab Invest. 1976 Apr;34(4):415–427. [PubMed] [Google Scholar]
- Sisson S. P., Vernier R. L. Methods for immunoelectron microscopy: Localization of antigens in rat kidney. J Histochem Cytochem. 1980 May;28(5):441–452. doi: 10.1177/28.5.6991591. [DOI] [PubMed] [Google Scholar]
- Taleporos P. Diethylene glycol distearate as an embedding medium for high resolution light microscopy. J Histochem Cytochem. 1974 Jan;22(1):29–34. doi: 10.1177/22.1.29. [DOI] [PubMed] [Google Scholar]
- Van Damme B. J., Fleuren G. J., Bakker W. W., Vernier R. L., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978 Apr;38(4):502–510. [PubMed] [Google Scholar]