Abstract
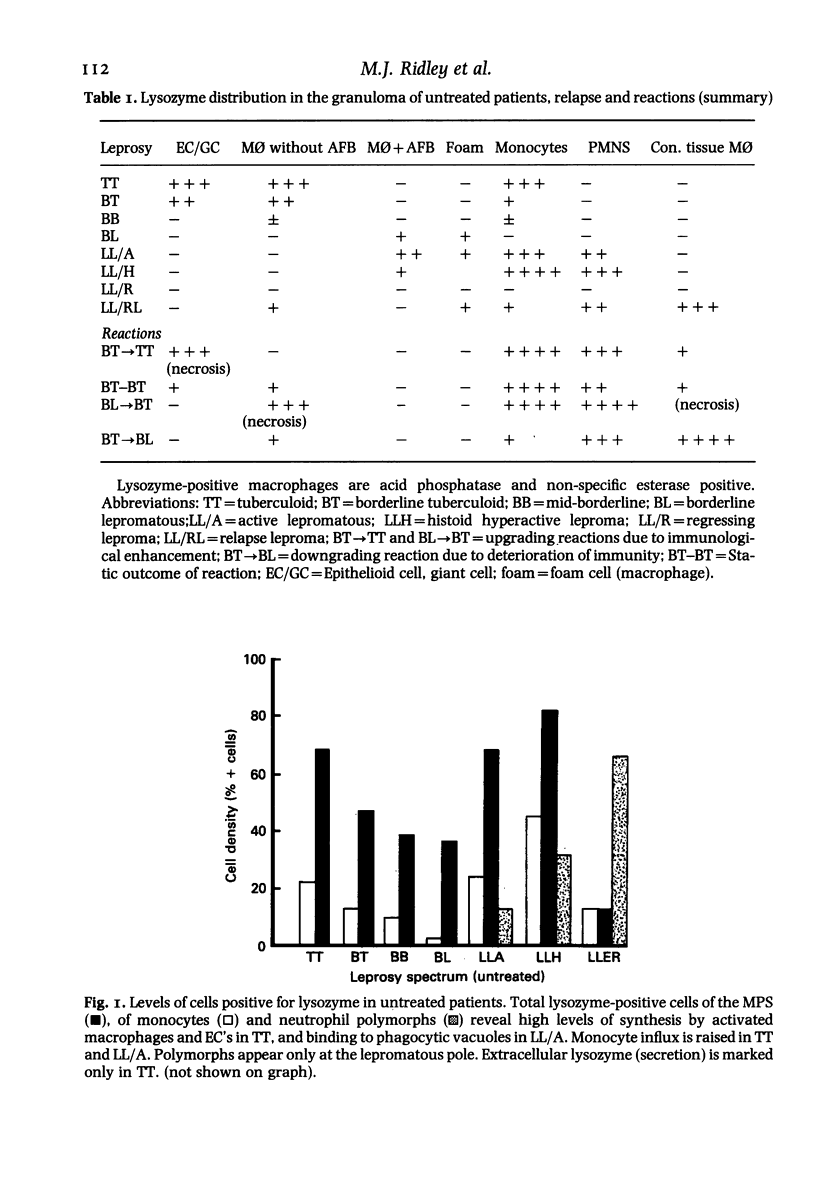
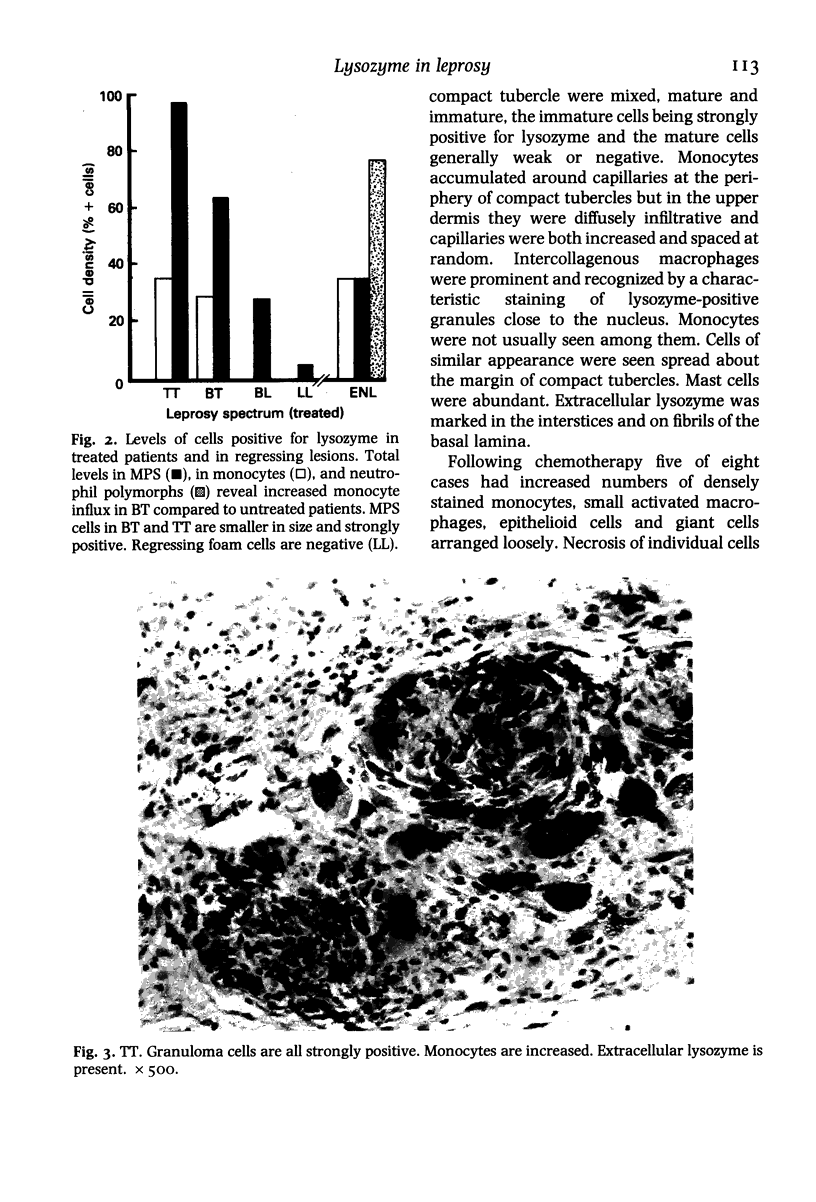
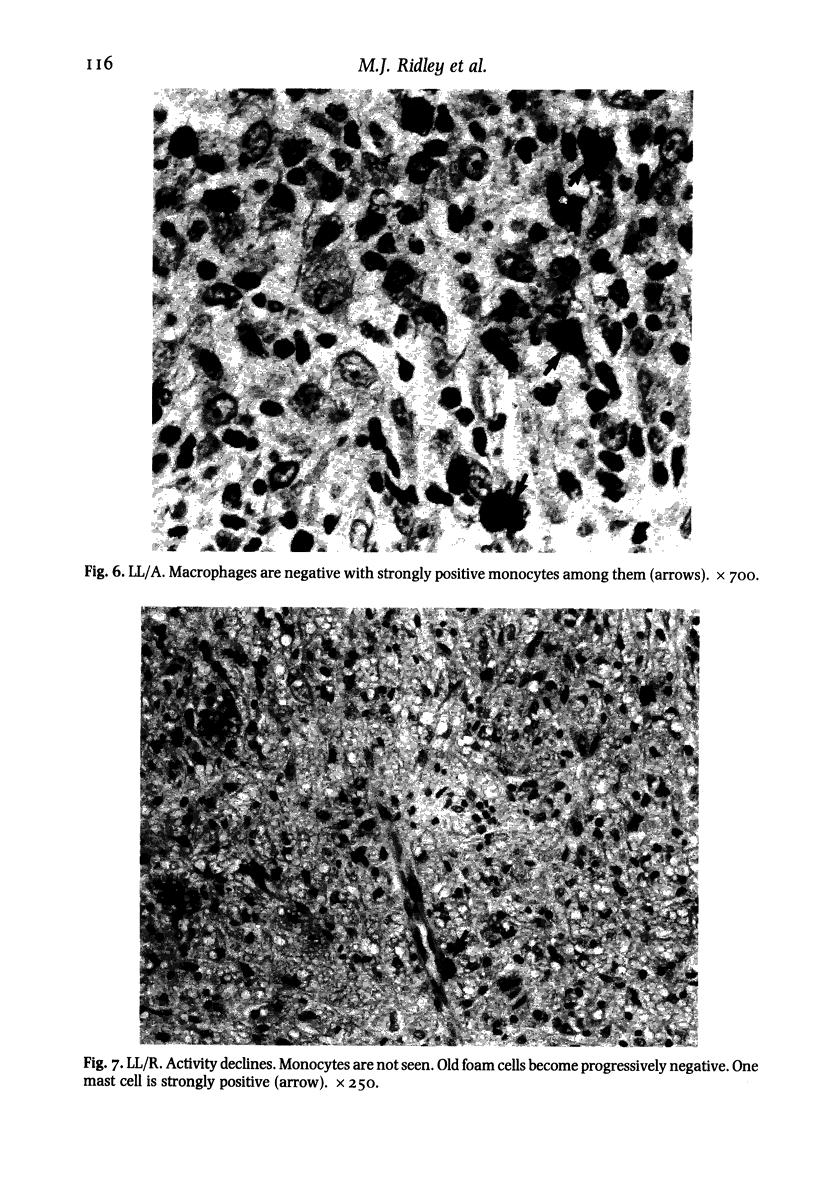
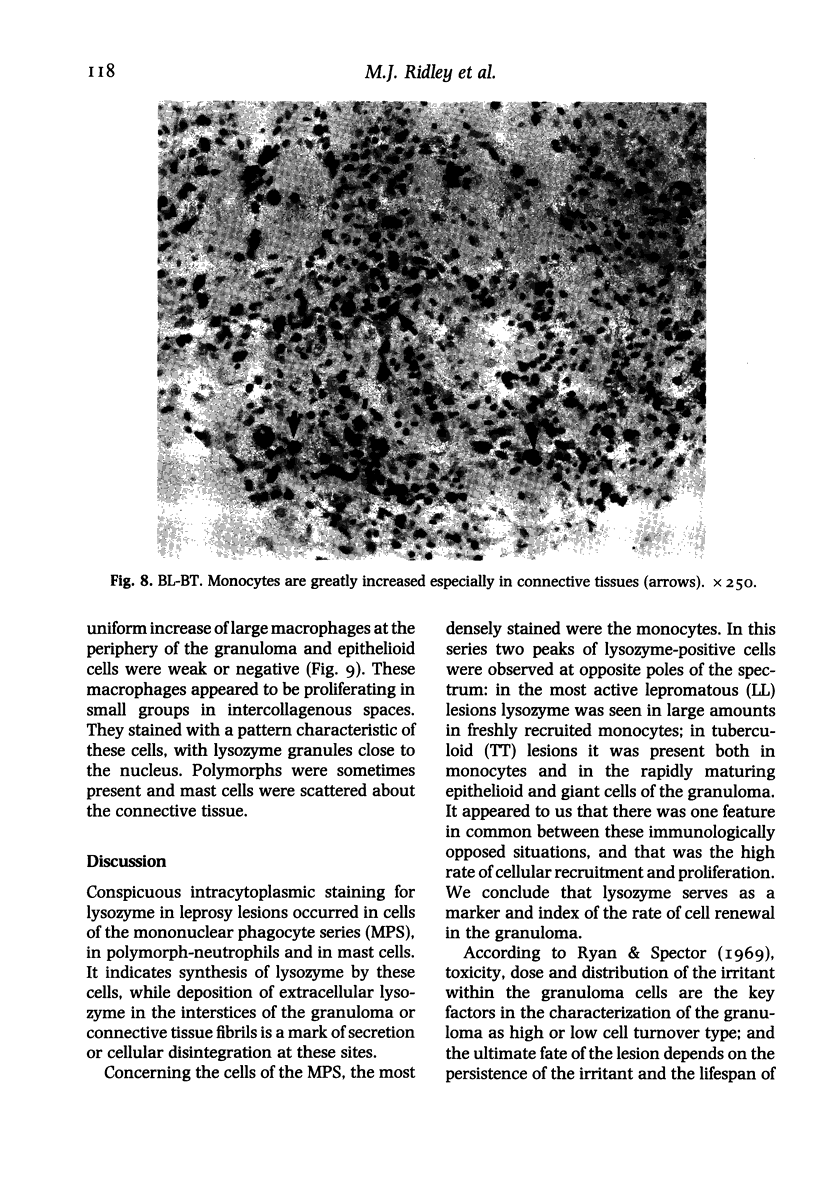
The levels and distribution of lysozyme-positive cells and exudate were studied in leprosy lesions through the spectrum, in untreated and treated patients, in relapse and in reactions. Altogether 124 skin biopsies were examined by the immunoperoxidase technique. Monocytes, neutrophil-polymorphs and mast cells were the most conspicuous cells seen. Lysozyme proved to be a useful means of indexing renewal of these cells in the lesions. Peak numbers of monocytes were seen in lesions of active lepromatous leprosy (LL) and of tuberculoid leprosy (TT), at poles of opposite immunological performance. In TT the stimulus for recruitment was delayed hypersensitivity (DH). A decline in DH from TT towards the middle of the spectrum, mid-borderline, was accompanied by a fall in monocyte level. Furthermore, reacting lesions due to enhanced DH also had increased numbers of monocytes. On the other hand reactions associated with immunological deterioration were similar to active lepromatous leprosy (LL) and monocyte influx was raised in response to the stimulus of free multiplication of bacilli in both cases. In TT delayed hypersensitivity acted also to promote the rapid transformation of monocytes to epithelioid and giant cells all of which were strongly positive for lysozyme. This was in contrast to much lower levels in histologically similar macrophage-epithelioid cells of BT granulomas. Lysozyme synthesis was not seen in macrophages after ingestion of M. leprae. Early foamy change was made conspicuous by lysozyme deposited in phagocytic vacuoles, but old foam cells in regressing lepromas were negative. Lysozyme bound to dead extracellular M. leprae but not to viable or intracellular organisms. Dead bacilli or immune complexes appeared to be the stimulus for neutrophil-polymorph recruitment, mainly in reactions.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carr I., Carr J., Trew J. A., Lobo A., Chattopadhyay P. K. Lysozyme production by a granuloma in vivo: output in blood and lymph in relation to ultrastructure and immunochemistry. J Pathol. 1980 Oct;132(2):105–119. doi: 10.1002/path.1711320203. [DOI] [PubMed] [Google Scholar]
- Fox R. A., MacSween J. M., Rajaraman K. Macrophage migration stimulation factor. Scand J Immunol. 1981 Oct;14(4):327–334. doi: 10.1111/j.1365-3083.1981.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindblom L. G., Jacobsen G. K., Jacobsen M. Immunohistochemical investigations of tumors of supposed fibroblastic-histiocytic origin. Hum Pathol. 1982 Sep;13(9):834–840. doi: 10.1016/s0046-8177(82)80080-4. [DOI] [PubMed] [Google Scholar]
- Lyberg T., Closs O., Prydz H. Effect of purified protein derivative and sonicates of Mycobacterium leprae and Mycobacterium bovis BCG on thromboplastin response in human monocytes in vitro. Infect Immun. 1982 Dec;38(3):855–859. doi: 10.1128/iai.38.3.855-859.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Y., Taylor C. R. The distribution of muramidase (lysozyme) in human tissues. J Clin Pathol. 1975 Feb;28(2):124–132. doi: 10.1136/jcp.28.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Ridley M. J., Heather C. J., Brown I., Willoughby D. A. Experimental epithelioid cell granulomas, tubercle formation and immunological competence: an ultrastructural analysis. J Pathol. 1983 Oct;141(2):97–112. doi: 10.1002/path.1711410202. [DOI] [PubMed] [Google Scholar]
- Ridley M. J., Ridley D. S., De Beer F. C., Pepys M. B. C-reactive protein and apoB containing lipoproteins are associated with Mycobacterium leprae in lesions of human leprosy. Clin Exp Immunol. 1984 Jun;56(3):545–552. [PMC free article] [PubMed] [Google Scholar]
- Ridley M. J., Ridley D. S., Turk J. L. Surface markers on lymphocytes and cells of the mononuclear phagocyte series in skin sections in leprosy. J Pathol. 1978 Jun;125(2):91–98. doi: 10.1002/path.1711250204. [DOI] [PubMed] [Google Scholar]
- Ridley M. J., Russell D. An immunoperoxidase study of immunological factors in high immune and low resistance granulomas in leprosy. J Pathol. 1982 Jun;137(2):149–157. doi: 10.1002/path.1711370208. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Spector W. G. Natural selection of long-lived macrophages in experimental granulomata. J Pathol. 1969 Oct;99(2):139–151. doi: 10.1002/path.1710990208. [DOI] [PubMed] [Google Scholar]
- Werb Z. How the macrophage regulates its extracellular environment. Am J Anat. 1983 Mar;166(3):237–256. doi: 10.1002/aja.1001660302. [DOI] [PubMed] [Google Scholar]