Abstract
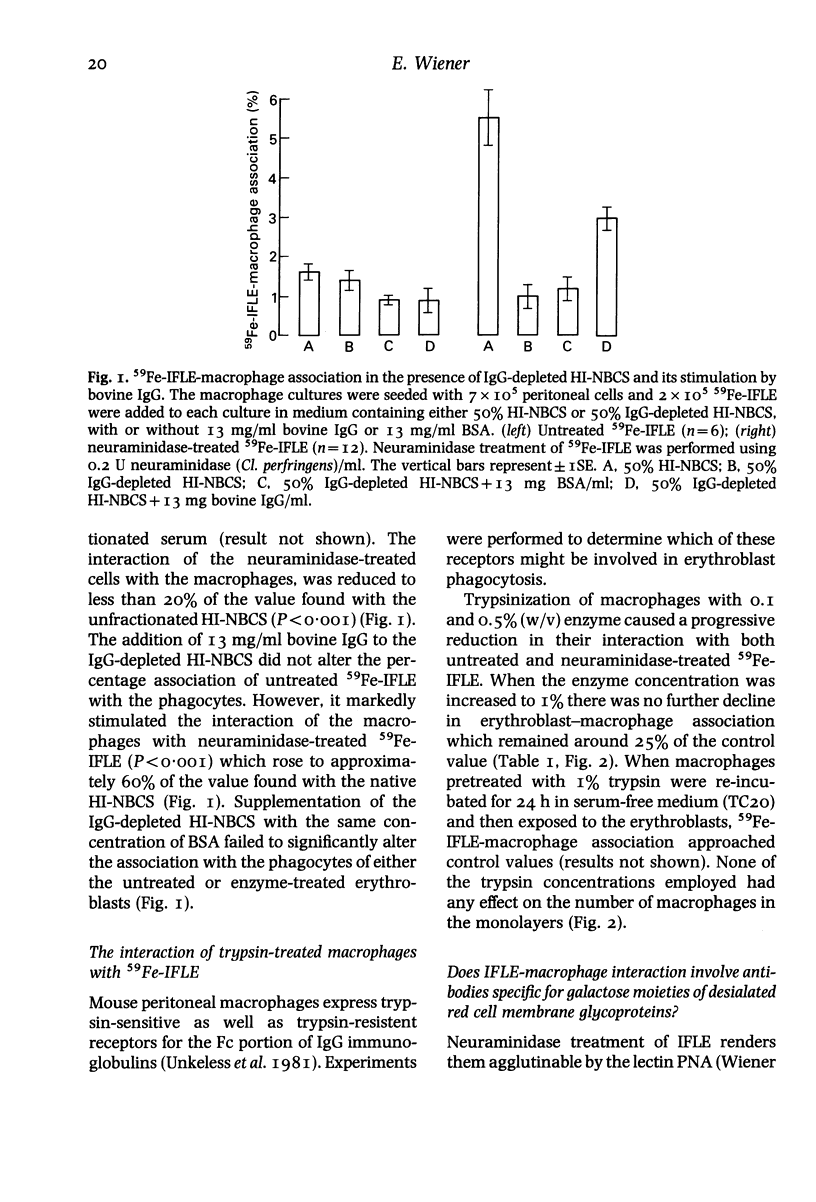
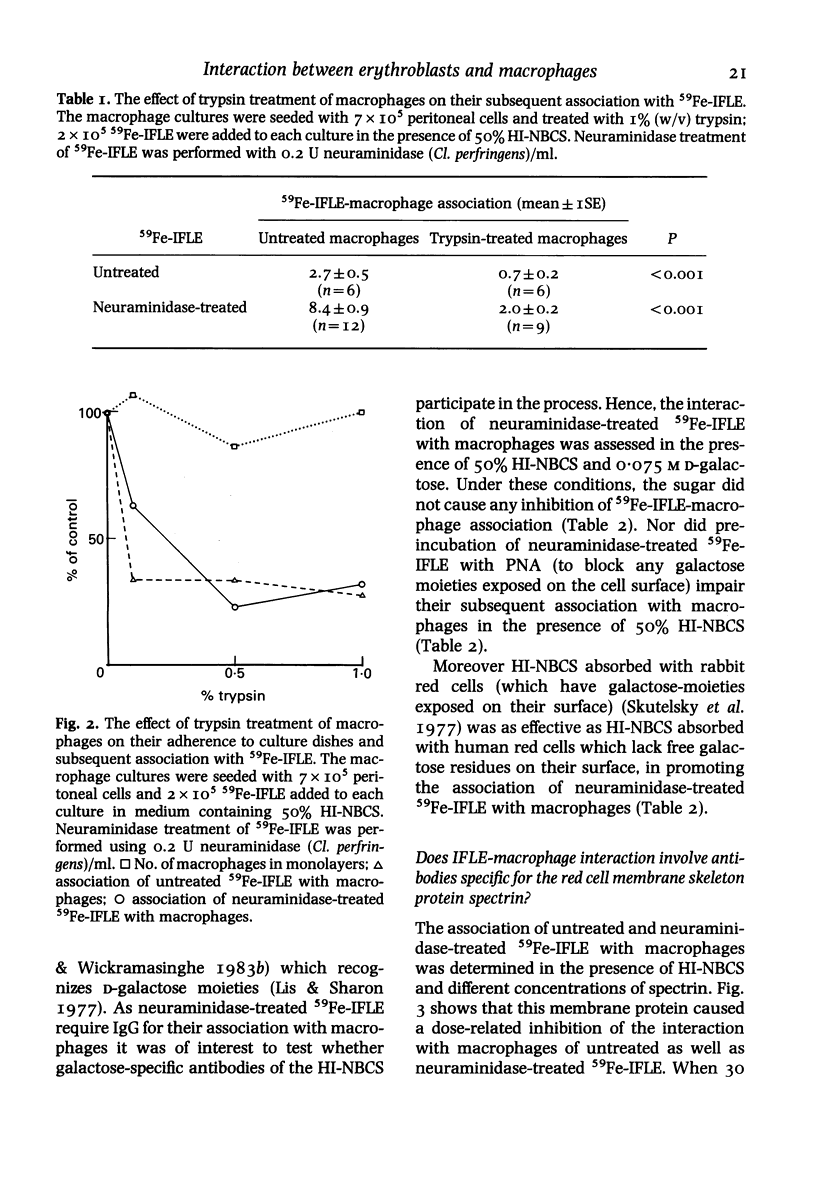
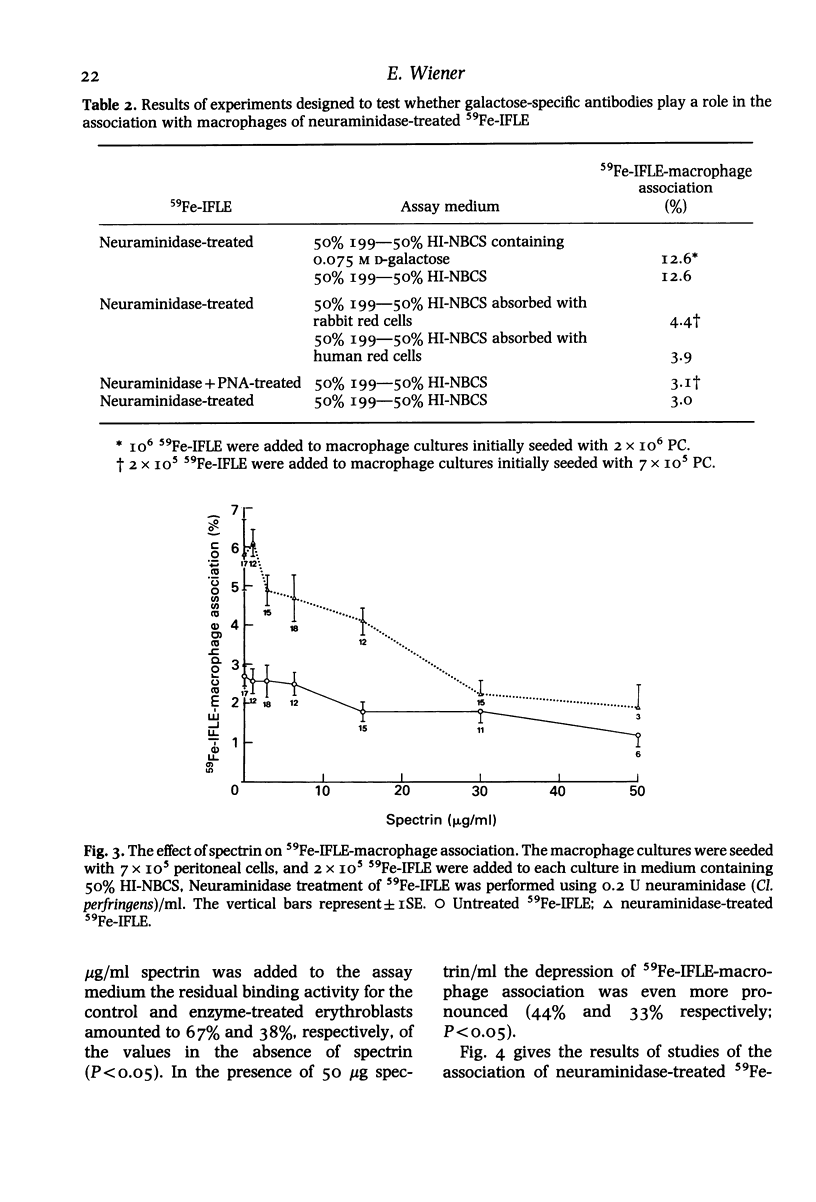
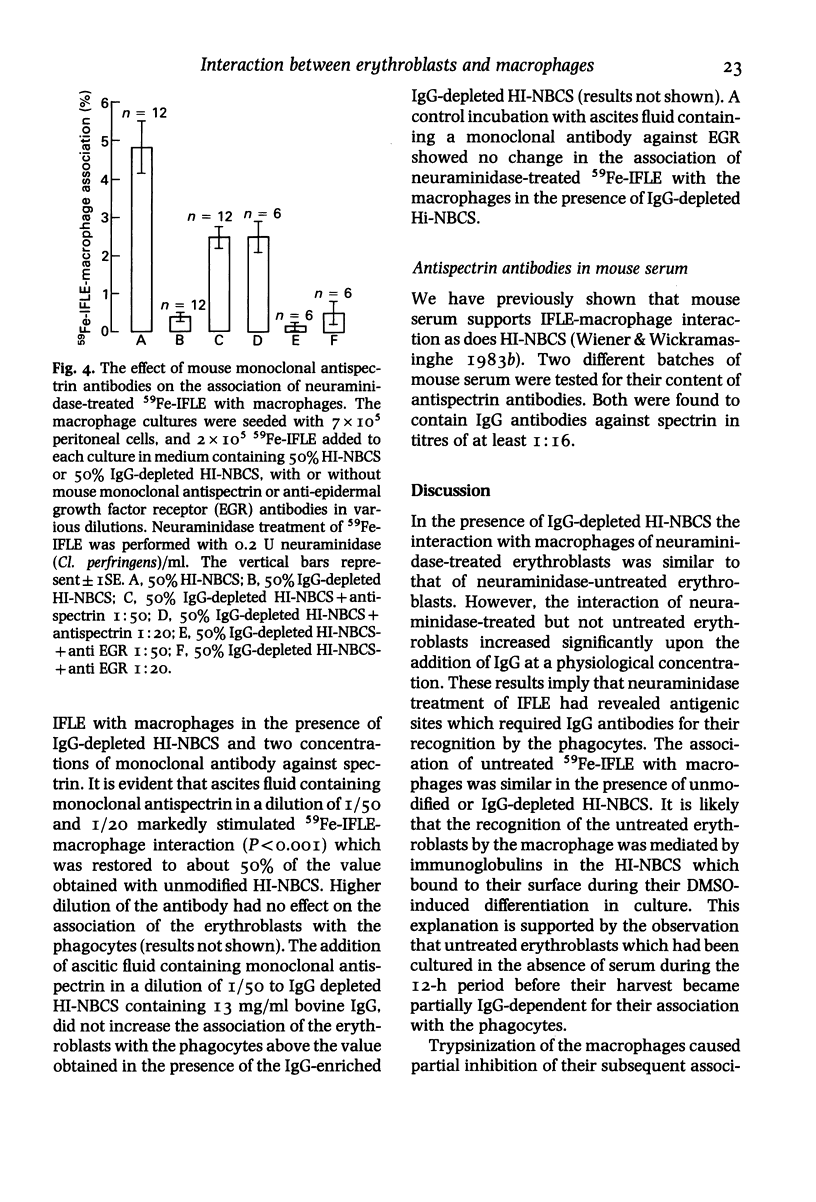
The nature of serum factors which participate in the interaction in vitro between dimethylsulphoxide-induced Friend leukaemia erythroblasts (IFLE) and syngeneic mouse peritoneal macrophages was investigated. When heat-inactivated newborn calf serum (HI-NBCS) was depleted of IgG its activity to promote the association of neuraminidase-treated 59Fe-labelled IFLE (59Fe-IFLE) with macrophages was markedly reduced but could be restored by the addition of bovine IgG. Trypsin treatment of macrophages caused incomplete inhibition of their subsequent association with both untreated and neuraminidase-treated 59Fe-IFLE in the presence of HI-NBCS. When spectrin, the major red cell cytoskeleton protein, was added to HI-NBCS there was a dose-related inhibition of the association with macrophages of both untreated and neuraminidase-treated 59Fe-IFLE. Moreover a mouse monoclonal antibody against spectrin promoted the interaction of neuraminidase-treated 59Fe-IFLE with macrophages. Mouse sera which supported the association of neuraminidase-treated 59Fe-IFLE with macrophages were found to contain anti-spectrin antibodies. These results suggest that IgG antibodies mediate the interaction between erythroblasts and macrophages via trypsin-sensitive and trypsin-resistant receptors on the macrophage surface and that at least some of the antibodies show specificity for spectrin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderman E. M., Fudenberg H. H., Lovins R. E. Isolation and characterization of an age-related antigen present on senescent human red blood cells. Blood. 1981 Aug;58(2):341–349. [PubMed] [Google Scholar]
- Cohen C. M. The molecular organization of the red cell membrane skeleton. Semin Hematol. 1983 Jul;20(3):141–158. [PubMed] [Google Scholar]
- Lutz H. U., Wipf G. Naturally occurring autoantibodies to skeletal proteins from human red blood cells. J Immunol. 1982 Apr;128(4):1695–1699. [PubMed] [Google Scholar]
- Newman S. L., Musson R. A., Henson P. M. Development of functional complement receptors during in vitro maturation of human monocytes into macrophages. J Immunol. 1980 Nov;125(5):2236–2244. [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Skutelsky E., Lotan R., Sharon N., Danon D. Distribution of the T-antigen on erythroid cell surfaces. Studies with peanut agglutinin, an anti-T specific lectin. Biochim Biophys Acta. 1977 Jun 2;467(2):165–174. doi: 10.1016/0005-2736(77)90193-6. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Fleit H., Mellman I. S. Structural Aspects and Heterogeneity of Immunoglobulin Fc Receptors. Adv Immunol. 1981;31:247–270. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Whitfield C. F., Mylin L. M., Goodman S. R. Species-dependent variations in erythrocyte membrane skeletal proteins. Blood. 1983 Mar;61(3):500–506. [PubMed] [Google Scholar]
- Wiener E., Wickramasinghe S. N. Impaired protein synthesis in erythroblasts enhances their phagocytosis by macrophages. Br J Haematol. 1983 Jan;53(1):117–124. doi: 10.1111/j.1365-2141.1983.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Wiener E., Wickramasinghe S. N. Interaction between erythroblasts and macrophages in vitro: effect of neuraminidase-treatment of erythroblasts and the role of serum factors. Br J Haematol. 1983 Oct;55(2):369–378. doi: 10.1111/j.1365-2141.1983.tb01258.x. [DOI] [PubMed] [Google Scholar]
- Wiener E., Wickramasinghe S. N. The interaction of DMSO-induced murine Friend leukaemia erythroblasts with cultured syngeneic mouse peritoneal macrophages: an in vitro model for the study of ineffective erythropoiesis. Br J Exp Pathol. 1982 Dec;63(6):586–593. [PMC free article] [PubMed] [Google Scholar]



