Abstract
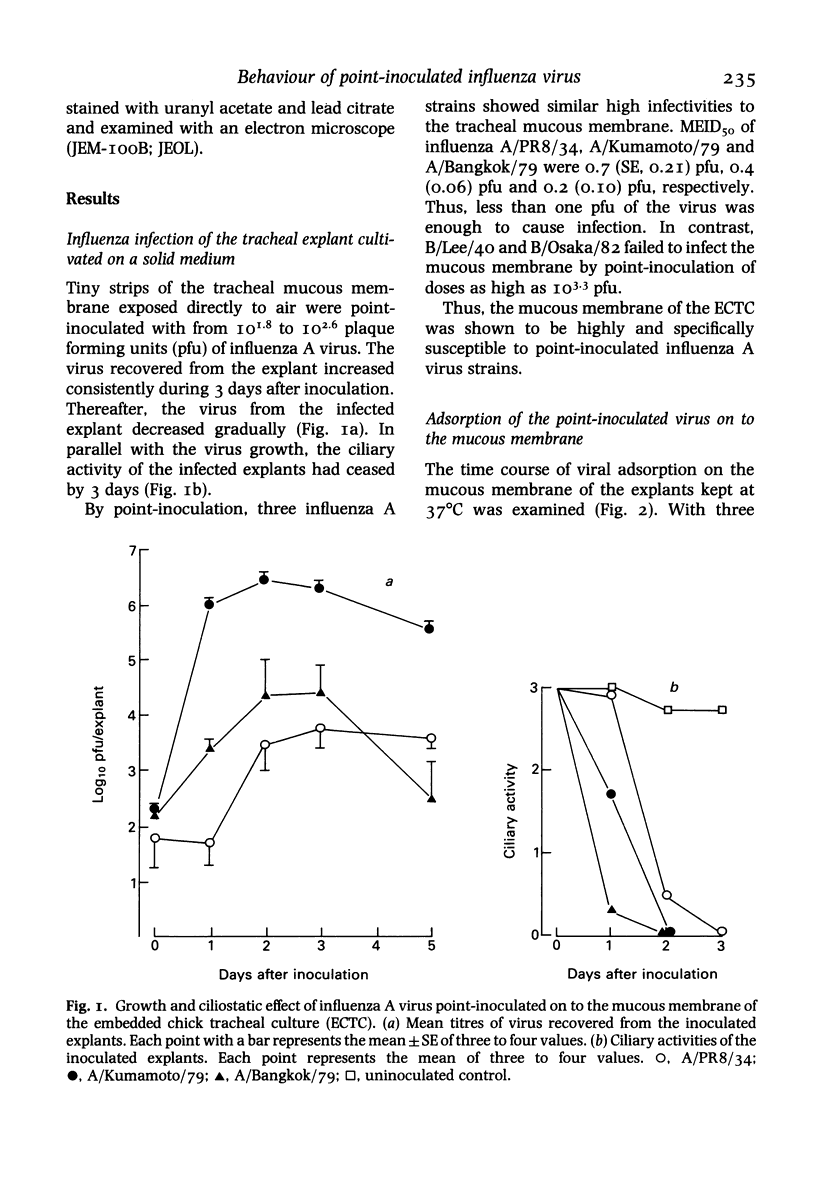
Influenza virus was point-inoculated on to the chick tracheal mucous membrane cultivated on a solid medium (L-15 agar medium) with a platinum microloop (ca 0.03 microliter of inoculum). Following the rapid adsorption of the inoculated virus on the mucosal surface, the explant was infected with a high efficiency. The 50% minimal explant infectious doses (MEID50) of influenza A virus strains (A/PR8/34, A/Kumamoto/79 and A/Bangkok/79) were less than one pfu. Influenza B virus (B/Lee/40) was also adsorbed to the mucous membrane but this virus failed to infect the chick tracheal mucous membrane (MEID50 greater than 10(3.3) pfu). By point-inoculation of virus on to the large tracheal explant (2 X 12 mm), it was possible to trace the behaviour of the infecting virus on the tracheal mucous membrane. Thus, influenza virus A/Kumamoto/79 was shown to be infective at the point-inoculated site by overcoming mucociliary clearance. Thereafter, depending on virus production and the ciliary activity of the infected epithelial cells, the infection spread rapidly to the laryngeal side but more slowly to the bronchial side of the tracheal explant.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaskovic P., Rhodes A. J., Labzoffsky N. A. Infection of chick embryo tracheal organ cultures with influenza A2 (Hong Kong) virus. I. Cytopathology, histopathology, immunofluorescence, hemadsorption, and titration of the released infectious progeny virus. Arch Gesamte Virusforsch. 1972;37(1):104–113. doi: 10.1007/BF01241156. [DOI] [PubMed] [Google Scholar]
- Gould E. A., Ratcliffe N. A., Basarab O., Smith H. Studies of the basis of localization of influenza virus in ferret organ cultures. Br J Exp Pathol. 1972 Feb;53(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Hara K., Beare A. S., Tyrrell D. A. Growth and pathogenicity of influenza viruses in organ cultures of ciliated epithelium. I. Experiments in ferret and human tissue. Arch Gesamte Virusforsch. 1974;44(3):227–236. doi: 10.1007/BF01240610. [DOI] [PubMed] [Google Scholar]
- Husseini R. H., Sweet C., Bird R. A., Collie M. H., Smith H. Distribution of viral antigen with the lower respiratory tract of ferrets infected with a virulent influenza virus: production and release of virus from corresponding organ cultures. J Gen Virol. 1983 Mar;64(Pt 3):589–598. doi: 10.1099/0022-1317-64-3-589. [DOI] [PubMed] [Google Scholar]
- Matsuyama T. Resistance of Bordetella pertussis phase I to mucociliary clearance by rabbit tracheal mucous membrane. J Infect Dis. 1977 Nov;136(5):609–616. doi: 10.1093/infdis/136.5.609. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Takino T. Scanning electronmicroscopic studies of Bordetella bronchiseptica on the rabbit tracheal mucosa. J Med Microbiol. 1980 Feb;13(1):159–161. doi: 10.1099/00222615-13-1-159. [DOI] [PubMed] [Google Scholar]
- Murakami T., Matsuyama T. Growth of Bacteroides fragilis inoculated on rabbit tracheal explant in an atmosphere environment. J Infect Dis. 1980 Sep;142(3):332–337. doi: 10.1093/infdis/142.3.332. [DOI] [PubMed] [Google Scholar]
- Murakami T., Matsuyama T., Shiraishi S., Hagihara B. Growth of Bacteroides fragilis in rabbit tracheal organ culture: anaerobiosis and tissue respiration. Infect Immun. 1981 Dec;34(3):1062–1064. doi: 10.1128/iai.34.3.1062-1064.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet C., Macartney J. C., Bird R. A., Cavanagh D., Collie M. H., Husseini R. H., Smith H. Differential distribution of virus and histological damage in the lower respiratory tract of ferrets infected with influenza viruses of differing virulence. J Gen Virol. 1981 May;54(Pt 1):103–114. doi: 10.1099/0022-1317-54-1-103. [DOI] [PubMed] [Google Scholar]
- Sweet C., Smith H. Pathogenicity of influenza virus. Microbiol Rev. 1980 Jun;44(2):303–330. doi: 10.1128/mr.44.2.303-330.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeura S., Aoki H., Tsurumi T., Nishiyama Y., Fujioka H., Yoshii S., Maeno K. Abortive infection of L cells by influenza B virus: defect in bud formation. Microbiol Immunol. 1984;28(4):427–437. doi: 10.1111/j.1348-0421.1984.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Tobita K., Sugiura A., Enomote C., Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975 Dec 30;162(1):9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- Yilma T., Zee Y. C., Osebold J. W. Immunofluorescence determination of the pathogenesis of infection with influenza virus in mice following exposure to aerosolized virus. J Infect Dis. 1979 Apr;139(4):458–464. doi: 10.1093/infdis/139.4.458. [DOI] [PubMed] [Google Scholar]