Abstract
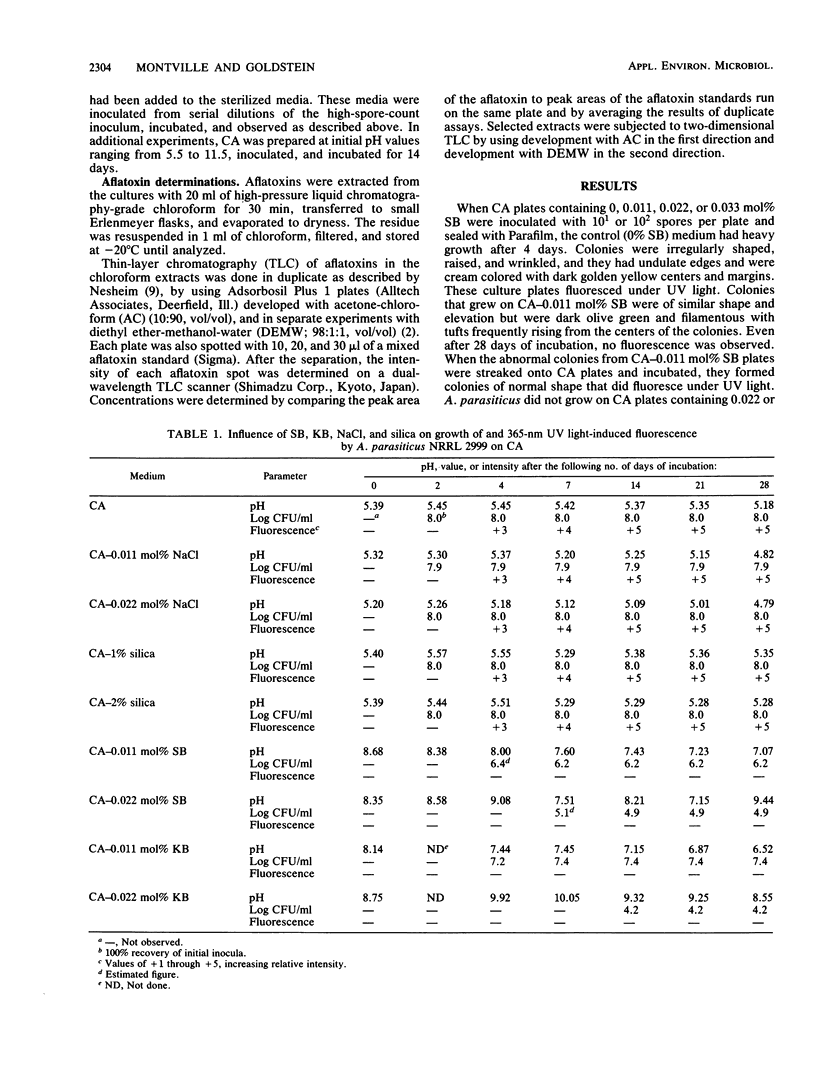
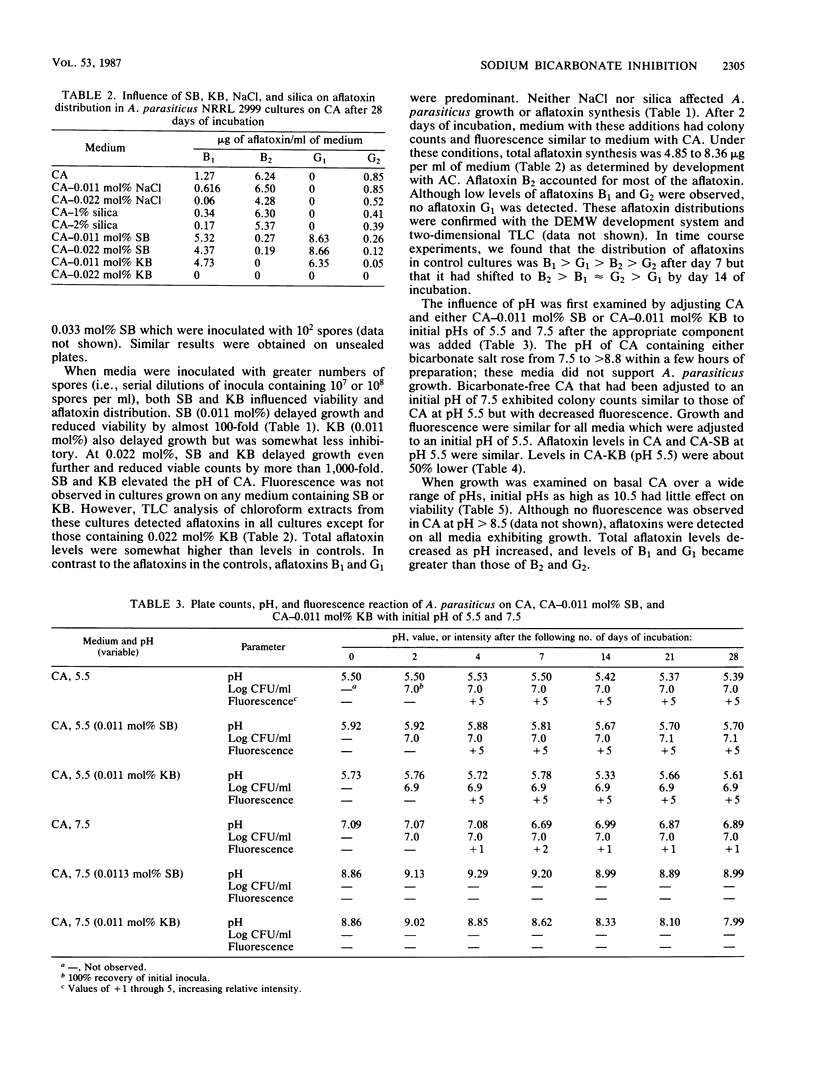
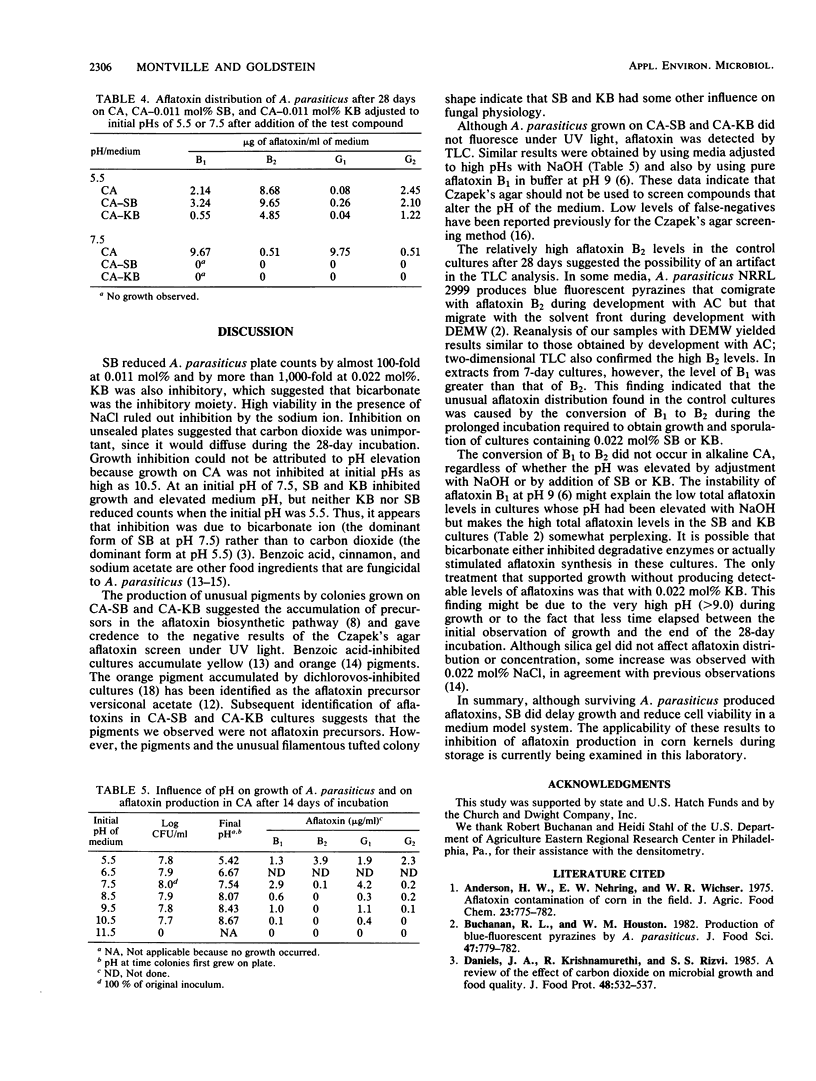
The potential of sodium bicarbonate to inhibit growth of and aflatoxin synthesis by Aspergillus parasiticus was examined in Czapek's agar (CA), a medium in which fluorescence under UV light indicates aflatoxin production. Incorporation of sodium bicarbonate (SB) into CA at 0.011, 0.022, and 0.033 mol% reduced cell viability 63-, 10(3)-, and greater than 10(7)-fold, respectively. Colonies resulting from surviving cells did not fluoresce under UV light, but thin-layer chromatography analysis of culture extracts detected aflatoxins. Potassium bicarbonate (KB) at 0.011 and 0.022 mol% produced inhibitory effects similar to those of SB, but NaCl and silica had no effect. After 7 days, control cultures had the normal aflatoxin distribution (B1 greater than G1 greater than B2 greater than G2), but this distribution shifted to B2 greater than B1 approximately equal to G2 greater than G1 during prolonged incubation. Cultures supplemented with SB and KB contained mostly aflatoxins B1 and G1 after 28 days. Both SB and KB raised the pH of CA to 7.5 to 8.5 at the time of growth. Culture growth on CA adjusted to pH 7.5 to 8.5 with NaOH was not inhibited but exhibited reduced fluorescence and elevated levels of aflatoxins B1 and G1. Thus, while bicarbonate inhibition of growth could not be attributed to pH elevation, the lack of culture fluorescence on CA-SB and CA-KB and the altered aflatoxin distribution were caused by the ability of SB and KB to elevate pH.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. W., Nehring E. W., Wichser W. R. Aflatoxin contamination of corn in the field. J Agric Food Chem. 1975 Jul-Aug;23(4):775–782. doi: 10.1021/jf60200a014. [DOI] [PubMed] [Google Scholar]
- Failla L. J., Lynn D., Niehaus W. G., Jr Correlation of Zn2+ content with aflatoxin content of corn. Appl Environ Microbiol. 1986 Jul;52(1):73–74. doi: 10.1128/aem.52.1.73-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S., Fennell D. I., Hesseltine C. W. Aflatoxin-producing strains of Aspergillus flavus detected by fluorescence of agar medium under ultraviolet light. Appl Microbiol. 1974 Jun;27(6):1118–1123. doi: 10.1128/am.27.6.1118-1123.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers K. E., Davis N. D., Diener U. L. Influence of atmospheric gases on aflatoxin production by Aspergillus flavus in peanuts. Phytopathology. 1967 Oct;57(10):1086–1090. [PubMed] [Google Scholar]
- Maggon K. K., Gupta S. K., Venkitasubramanian T. A. Biosynthesis of aflatoxins. Bacteriol Rev. 1977 Dec;41(4):822–855. doi: 10.1128/br.41.4.822-855.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders T. H., Davis N. D., Diener U. L. Effect of carbon dioxide, temperature, and relative humidity on production of aflatoxin in peanuts. J Am Oil Chem Soc. 1968 Oct;45(10):683–685. doi: 10.1007/BF02541257. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Cole R. J., Grigsby R. D., Hein H., Jr Inhibition of aflatoxin production and tentative identification of an aflatoxin intermediate "versiconal acetate" from treatment with dichlorvos. Appl Microbiol. 1974 Feb;27(2):394–399. doi: 10.1128/am.27.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uraih N., Chipley J. R. Effects of various acids and salts on growth and aflatoxin production by Aspergillus flavus NRRL 3145. Microbios. 1976;17(67):51–59. [PubMed] [Google Scholar]
- Valcarcel R., Bennett J. W., Vitanza J. Effect of selected inhibitors on growth, pigmentation, and aflatoxin production by Aspergillus parasiticus. Mycopathologia. 1986 Apr;94(1):7–10. doi: 10.1007/BF00437255. [DOI] [PubMed] [Google Scholar]
- Wicklow D. T., Shotwell O. L., Adams G. L. Use of aflatoxin-producing ability medium to distinguish aflatoxin-producing strains of Aspergillus flavus. Appl Environ Microbiol. 1981 Mar;41(3):697–699. doi: 10.1128/aem.41.3.697-699.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Huang L. H., Jay E. Survival of Aspergillus flavus and Fusarium moniliforme in high-moisture corn stored under modified atmospheres. Appl Microbiol. 1975 Oct;30(4):592–595. doi: 10.1128/am.30.4.592-595.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R. C., Hsieh D. P. Step of dichlorvos inhibition in the pathway of aflatoxin biosynthesis. Appl Microbiol. 1974 Jul;28(1):52–57. doi: 10.1128/am.28.1.52-57.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


