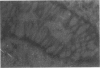
Abstract
We measured the permeability of normal, adenomatous, colitic and malignant large bowel epithelial cells by immersing fragments of large bowel mucosa in radiolabelled inulin and comparing autoradiograph grain density inside and outside cells after incubation. All the carcinomas studied showed extensive uptake of inulin within 5 min, while normal, adenomatous and colitic epithelial cells completely excluded inulin for 30 min. We found no difference in the proportion of epithelial cells incorporating uridine into RNA in carcinomatous and normal mucosa, and this suggests that the increased inulin permeability of carcinoma cell membranes was not due to leakage into non-viable cells. Experiments with cytochalasin B also showed that increased pinocytosis by carcinoma cells could not account for the difference. The relative impermeability of adenomatous and colitic cells suggests that increased permeability is not caused by increased proliferation. The consistent finding of increased permeability in the plasma membranes of carcinoma cells suggests that this may be more than an epiphenomenon of malignancy. It also suggests that measurement of cell permeability may have a role in distinguishing malignant from benign epithelial neoplasms.
Full text
PDF

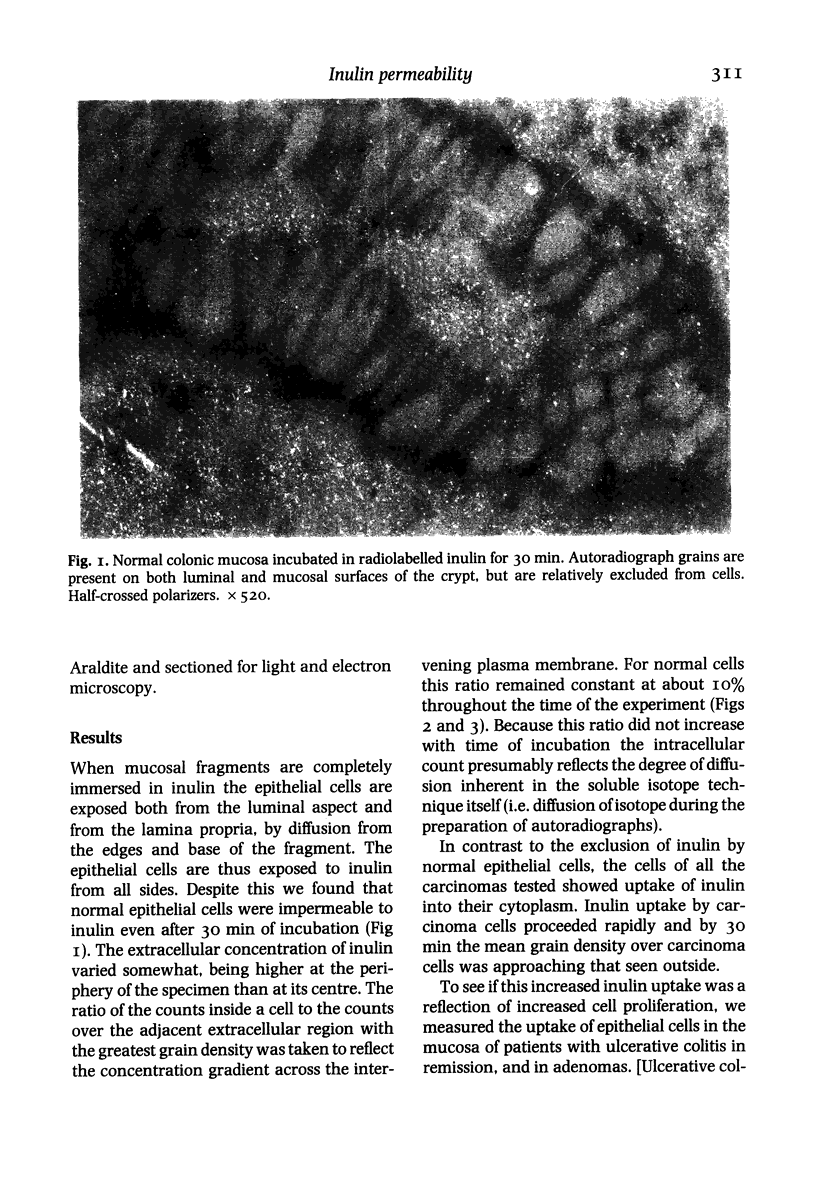
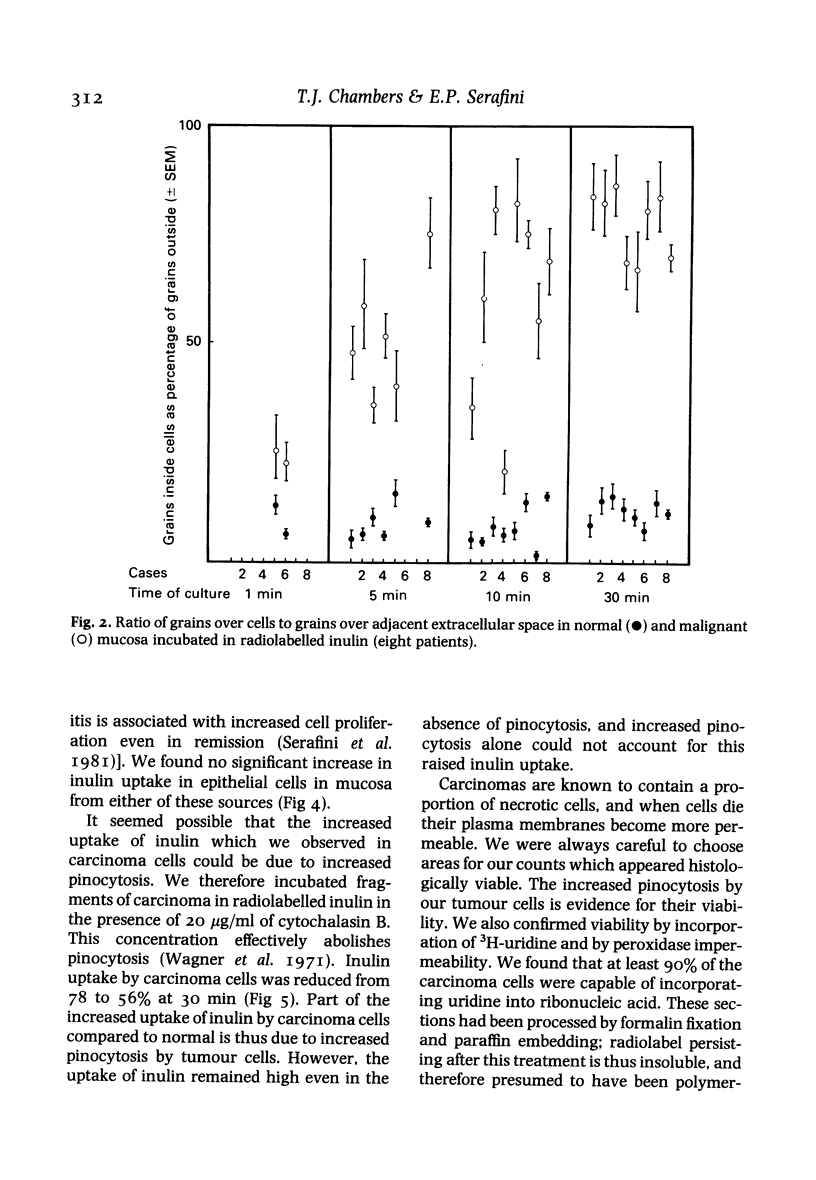
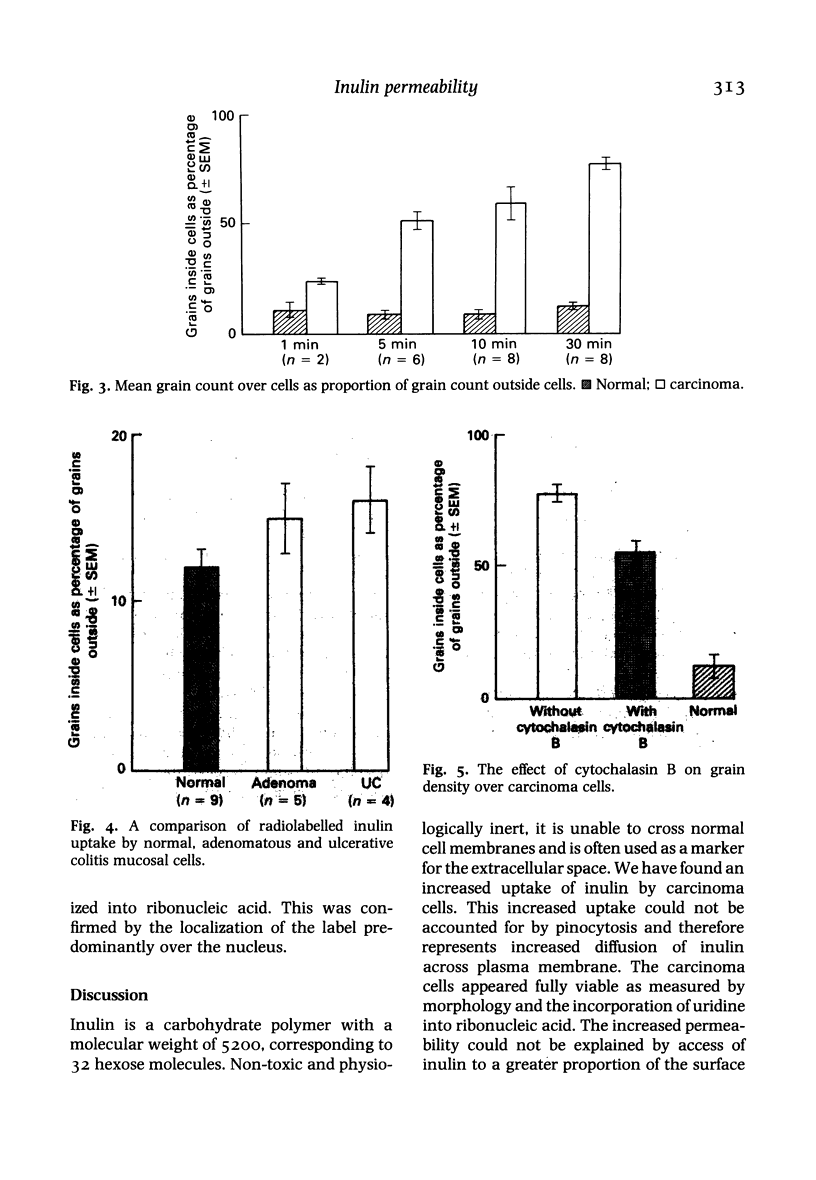


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashall F., Bramwell M. E., Harris H. A new marker for human cancer cells. 1 The Ca antigen and the Ca1 antibody. Lancet. 1982 Jul 3;2(8288):1–6. doi: 10.1016/s0140-6736(82)91150-3. [DOI] [PubMed] [Google Scholar]
- BOSCH L. The release of enzymes from ascites-tumour cells into the extracellular medium. Biochim Biophys Acta. 1958 Nov;30(2):444–445. doi: 10.1016/0006-3002(58)90083-0. [DOI] [PubMed] [Google Scholar]
- Caruthers J. M., Bonneville M. A. Luminal plasma membrane alterations in bladder cancer. Invest Urol. 1980 Mar;17(5):364–372. [PubMed] [Google Scholar]
- Cone C. D., Jr Ionically mediated induction of mitogenesis in CNS neurons. Ann N Y Acad Sci. 1980;339:115–131. doi: 10.1111/j.1749-6632.1980.tb15973.x. [DOI] [PubMed] [Google Scholar]
- Hill M. J., Morson B. C., Bussey H. J. Aetiology of adenoma--carcinoma sequence in large bowel. Lancet. 1978 Feb 4;1(8058):245–247. doi: 10.1016/s0140-6736(78)90487-7. [DOI] [PubMed] [Google Scholar]
- Morson B. C., Pang L. S. Rectal biopsy as an aid to cancer control in ulcerative colitis. Gut. 1967 Oct;8(5):423–434. doi: 10.1136/gut.8.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Mendoza S. Monovalent ion fluxes and the control of cell proliferation in cultured fibroblasts. Ann N Y Acad Sci. 1980;339:175–190. doi: 10.1111/j.1749-6632.1980.tb15977.x. [DOI] [PubMed] [Google Scholar]
- Serafini E. P., Kirk A. P., Chambers T. J. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981 Aug;22(8):648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU R. Leakage of enzymes from ascites tumor cells. Cancer Res. 1959 Dec;19:1217–1222. [PubMed] [Google Scholar]