Abstract
The route of excretion of lipopolysaccharide (LPS) and its possible degradation in vivo was studied in rats using biosynthetically radiolabelled LPS from Salmonella abortus equi, carrying 3H activity exclusively in fatty acids and 14C activity in fatty acids and sugars. Following intravenous injection of the above LPS in AS2 rats with or without anaesthesia, excretion of radioactivity occurred mainly in the faeces and to smaller extent in urine. The rate of excretion was slow, a large part of the radioactivity being still present in the liver after 14 days. In faeces the percent recovery of 3H (18%) was lower than that of 14C (32%) suggesting loss of tritium activity and thereby of fatty acids from the excreted LPS. A similar loss of tritium was also found in the material remaining in the liver and spleen 14 days after LPS administration. In urine the material recovered during 14 days (about 7% of injected) was different from the original LPS, 70% of 14C activity being dialysable and practically all 3H activity being volatile. Similar results were also obtained following administration of the LPS intraperitoneally under anaesthesia. However, when the LPS was administered intraperitoneally without anaesthesia, in the majority of the animals, 90% of 14C and 54% of 3H was excreted in faeces within 3 days, suggesting that both route of administration and use of anaesthesia during injection influence the subsequent rate of excretion of LPS.
Full text
PDF

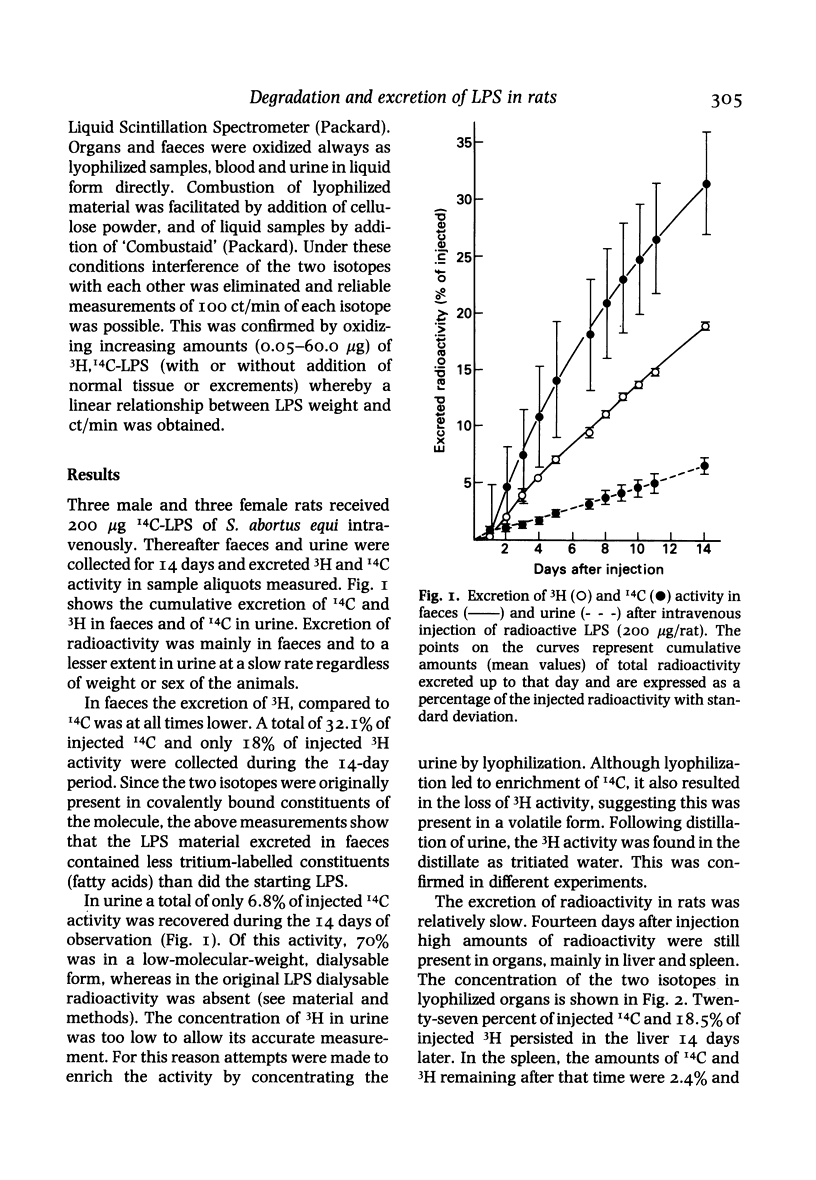
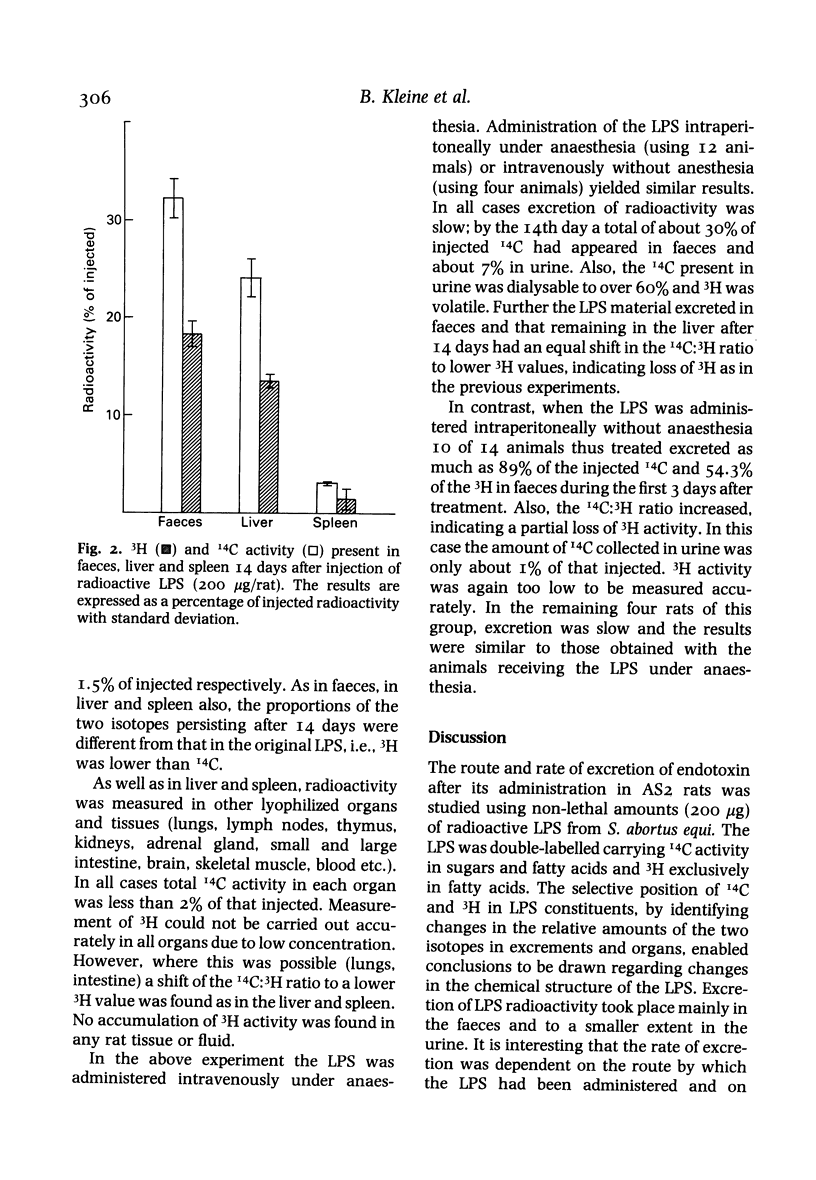


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E., Jr, Corwin L. M. The essential role of the liver in detoxification of endotoxin. Ann N Y Acad Sci. 1966 Jun 30;133(2):668–684. doi: 10.1111/j.1749-6632.1966.tb52397.x. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Bøg-Hansen T. C., Back U., Galanos C. Interaction of lipopolysaccharides with plasma high-density lipoprotein in rats. Infect Immun. 1980 May;28(2):373–380. doi: 10.1128/iai.28.2.373-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Freudenberg N., Galanos C. Time course of cellular distribution of endotoxin in liver, lungs and kidneys of rats. Br J Exp Pathol. 1982 Feb;63(1):56–65. [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Lüderitz O., Westphal O. Biochemical studies on lipopolysaccharides of Salmonella R mutants. 6. Investigations on the structure of the lipid A component. Eur J Biochem. 1969 Jan;7(3):370–379. doi: 10.1111/j.1432-1033.1969.tb19618.x. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Munford R. S. Enzymatic deacylation of the lipid A moiety of Salmonella typhimurium lipopolysaccharides by human neutrophils. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6671–6675. doi: 10.1073/pnas.80.21.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A., Goralnick S., Osborn M. J. Isolation from human serum of an inactivator of bacterial lipopolysaccharide. Am J Pathol. 1977 Sep;88(3):559–574. [PMC free article] [PubMed] [Google Scholar]
- KEENE W. R., LANDY M., SHEAR M. J. Inactivation of endotoxin by a humoral component. VII. Enzymatic degradation of endotoxin by blood plasma. J Clin Invest. 1961 Feb;40:302–310. doi: 10.1172/JCI104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. E., Kane M. A., Frank M. M. Host defense against bacterial endotoxemia-contribution of the early and late components of complement to detoxification. J Immunol. 1972 Oct;109(4):893–895. [PubMed] [Google Scholar]
- NETER E., WESTPHAL O., LUDERITZ O., GORZYNSKI E. A., EICHENBERGER E. Studies of enterobacterial lipopolysaccharides; effects of heat and chemicals on erythrocyte-modifying, antigenic, toxic and pyrogenic properties. J Immunol. 1956 May;76(5):377–385. [PubMed] [Google Scholar]
- Rudbach J. A., Anacker R. L., Haskins W. T., Johnson A. G., Milner K. C., Ribi E. Physical aspects of reversible inactivation of endotoxin. Ann N Y Acad Sci. 1966 Jun 30;133(2):629–643. doi: 10.1111/j.1749-6632.1966.tb52394.x. [DOI] [PubMed] [Google Scholar]


