Abstract
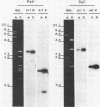
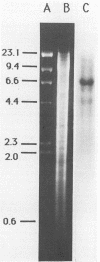
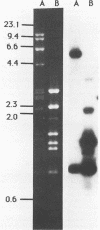
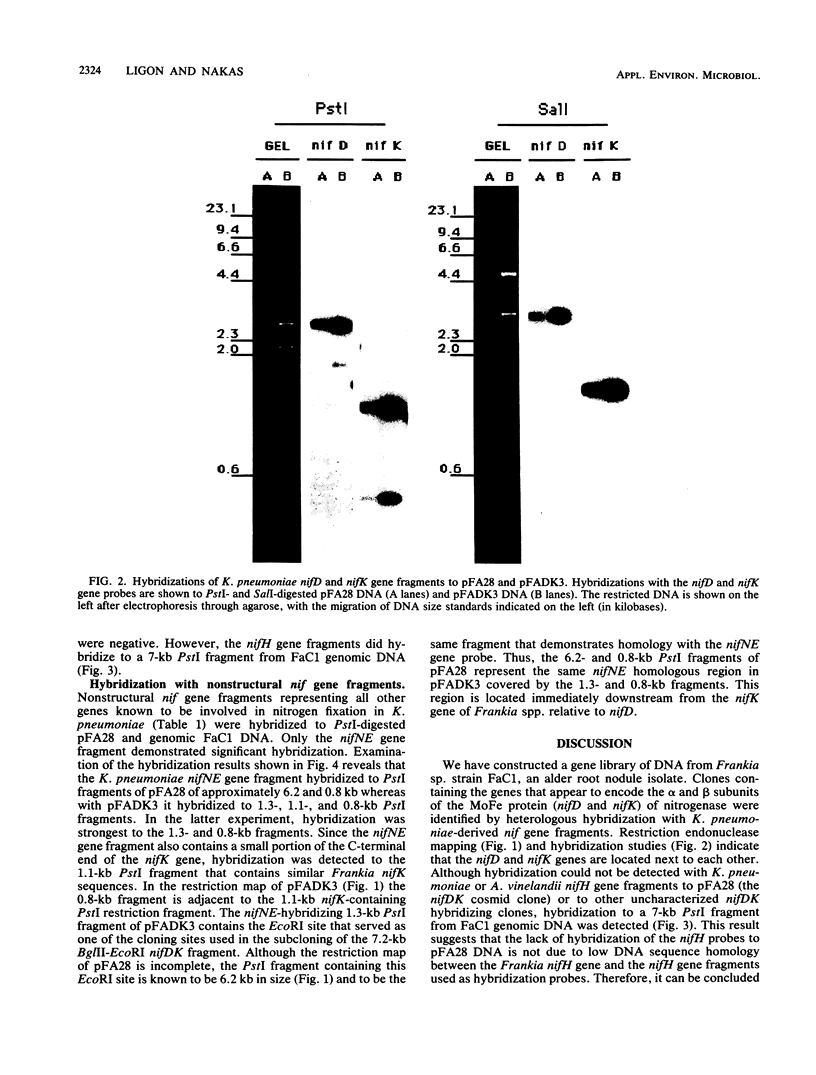
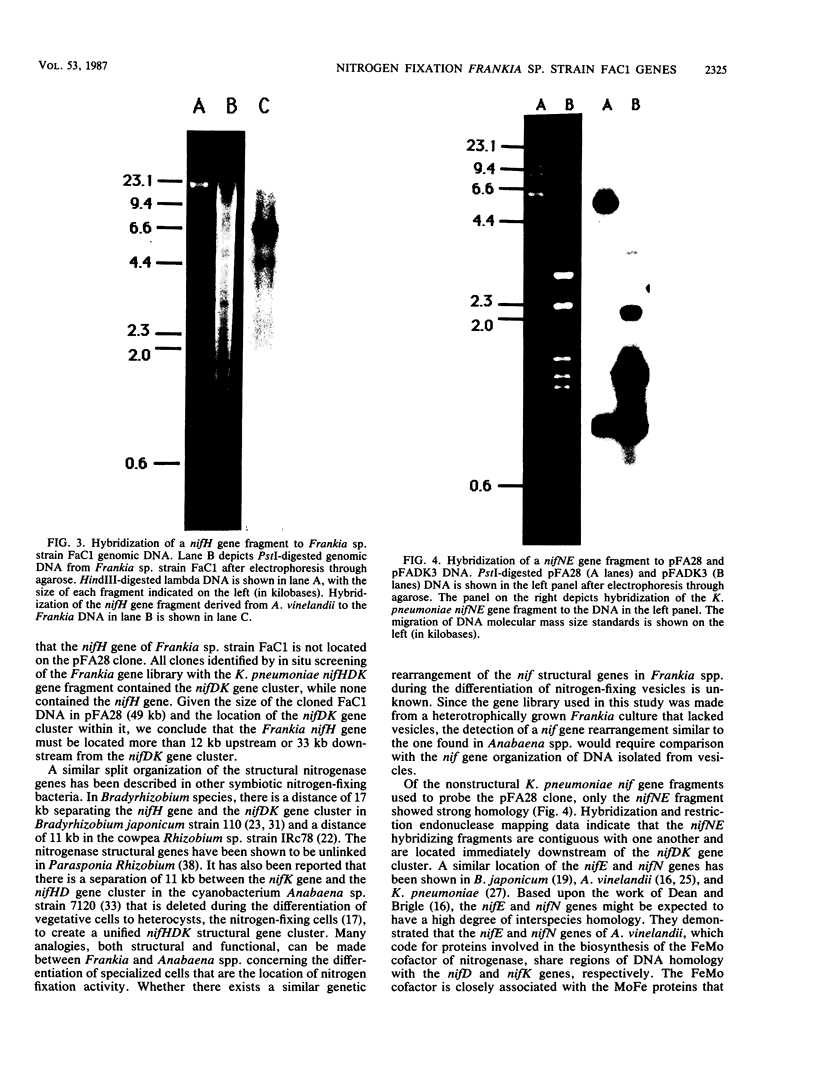
Genomic DNA was isolated from Frankia sp. strain FaC1, an Alnus root nodule endophyte, and used to construct a genomic library in the cosmid vector pHC79. The genomic library was screened by in situ colony hybridization to identify clones of Frankia nitrogenase (nif) genes based on DNA sequence homology to structural nitrogenase genes from Klebsiella pneumoniae. Several Frankia nif clones were isolated, and hybridization with individual structural nitrogenase gene fragments (nifH, nifD, and nifK) from K. pneumoniae revealed that they all contain the nifD and nifK genes, but lack the nifH gene. Restriction endonuclease mapping of the nifD and nifK hybridizing region from one clone revealed that the nifD and nifK genes in Frankia sp. are contiguous, while the nifH gene is absent from a large region of DNA on either side of the nifDK gene cluster. Additional hybridizations with gene fragments derived from K. pneumoniae as probes and containing other genes involved in nitrogen fixation demonstrated that the Frankia nifE and nifN genes, which play a role in the biosynthesis of the iron-molybdenum cofactor, are located adjacent to the nifDK gene cluster.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avtges P., Scolnik P. A., Haselkorn R. Genetic and physical map of the structural genes (nifH,D,K) coding for the nitrogenase complex of Rhodopseudomonas capsulata. J Bacteriol. 1983 Oct;156(1):251–256. doi: 10.1128/jb.156.1.251-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D. R., Buchholz S. E., Hanna D. G. Identification of frankia strains by two-dimensional polyacrylamide gel electrophoresis. Appl Environ Microbiol. 1984 Mar;47(3):489–494. doi: 10.1128/aem.47.3.489-494.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D. R. Isolation of frankia strains from alder actinorhizal root nodules. Appl Environ Microbiol. 1982 Aug;44(2):461–465. doi: 10.1128/aem.44.2.461-465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Rizzo T. M., Bott K. F. Molecular cloning of nif DNA from Azotobacter vinelandii. J Bacteriol. 1985 Apr;162(1):21–28. doi: 10.1128/jb.162.1.21-28.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Callaham D., Deltredici P., Torrey J. G. Isolation and Cultivation in vitro of the Actinomycete Causing Root Nodulation in Comptonia. Science. 1978 Feb 24;199(4331):899–902. doi: 10.1126/science.199.4331.899. [DOI] [PubMed] [Google Scholar]
- Cannon F. C., Riedel G. E., Ausubel F. M. Overlapping sequences of Klebsiella pneumoniae nifDNA cloned and characterized. Mol Gen Genet. 1979 Jul 2;174(1):59–66. doi: 10.1007/BF00433306. [DOI] [PubMed] [Google Scholar]
- Dean D. R., Brigle K. E. Azotobacter vinelandii nifD- and nifE-encoded polypeptides share structural homology. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5720–5723. doi: 10.1073/pnas.82.17.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza K., Fuhrmann M., Hahn M., Regensburger B., Hennecke H. In Rhizobium japonicum the nitrogenase genes nifH and nifDK are separated. J Bacteriol. 1983 Aug;155(2):915–918. doi: 10.1128/jb.155.2.915-918.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983 Nov;10(3):296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- MacNeil T., MacNeil D., Roberts G. P., Supiano M. A., Brill W. J. Fine-structure mapping and complementation analysis of nif (nitrogen fixation) genes in Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):253–266. doi: 10.1128/jb.136.1.253-266.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand P., Simonet P., Butour J. L., Rosenberg C., Moiroud A., Lalonde M. Plasmids in Frankia sp. J Bacteriol. 1983 Jul;155(1):32–35. doi: 10.1128/jb.155.1.32-35.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noti J. D., Folkerts O., Turken A. N., Szalay A. A. Organization and characterization of genes essential for symbiotic nitrogen fixation from Bradyrhizobium japonicum I110. J Bacteriol. 1986 Sep;167(3):774–783. doi: 10.1128/jb.167.3.774-783.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiviger B., Franche C., Lutfalla G., Rice D., Haselkorn R., Elmerich C. Cloning of a nitrogen fixation (nif) gene cluster of Azospirillum brasilense. Biochimie. 1982 Jul;64(7):495–502. doi: 10.1016/s0300-9084(82)80165-x. [DOI] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Riedel G. E., Ausubel F. M., Cannon F. C. Physical map of chromosomal nitrogen fixation (nif) genes of Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2866–2870. doi: 10.1073/pnas.76.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., Brill W. J. Genetics and regulation of nitrogen fixation. Annu Rev Microbiol. 1981;35:207–235. doi: 10.1146/annurev.mi.35.100181.001231. [DOI] [PubMed] [Google Scholar]
- Robson R., Kennedy C., Postgate J. Progress in comparative genetics of nitrogen fixation. Can J Microbiol. 1983 Aug;29(8):954–967. doi: 10.1139/m83-152. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. F., Rolfe B. G., Shine J. Nitrogenase structural genes are unlinked in the nonlegume symbiont Parasponia rhizobium. DNA. 1983;2(2):141–148. doi: 10.1089/dna.1983.2.141. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tjepkema J. D., Ormerod W., Torrey J. G. Factors affecting vesicle formation and acetylene reduction (nitrogenase activity) in Frankia sp. CpI1. Can J Microbiol. 1981 Aug;27(8):815–823. doi: 10.1139/m81-126. [DOI] [PubMed] [Google Scholar]