Abstract
Mice infected intracerebrally (i.c.) with wild-type (wt) VSV or temperature-sensitive (ts) mutants, ts 11, ts 22, ts 31 and ts 41, were studied for the development of histopathological lesions in the central nervous system (CNS). Mice infected i.c. with wt VSV exhibited histopathological lesions consisting principally of occasional foci of perivascular mononuclear cell infiltration and rare foci of necrosis. All wt VSV infected mice died within 2 days of i.c. inoculation. In contrast, mice infected i.c. with ts 22 and ts 31 developed spongiform lesions limited to the grey matter of the spinal cord beginning 4 days after inoculation. The spongiform lesions rapidly spread to involve the entire grey matter of the spinal cord by 5-7 days after infection. Vacuolar changes were restricted principally to neuronal processes and astrocytes. Ts 22 and ts 31 infected mice developed neurological illness beginning 4 days after infection and the majority of mice died by 7 days after infection. Mice infected with ts 11 and ts 41, on the other hand, remained clinically well and were devoid of neuro-pathological lesions at 4 and 8 days after infection.
Full text
PDF

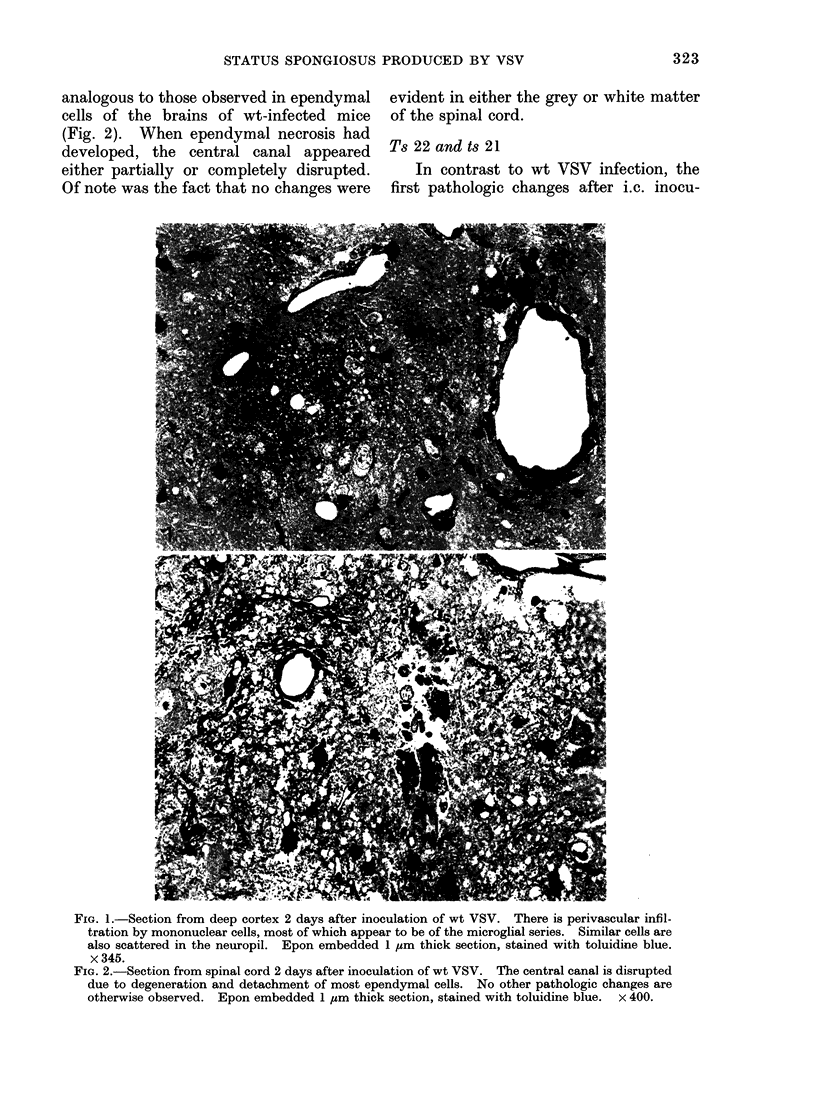
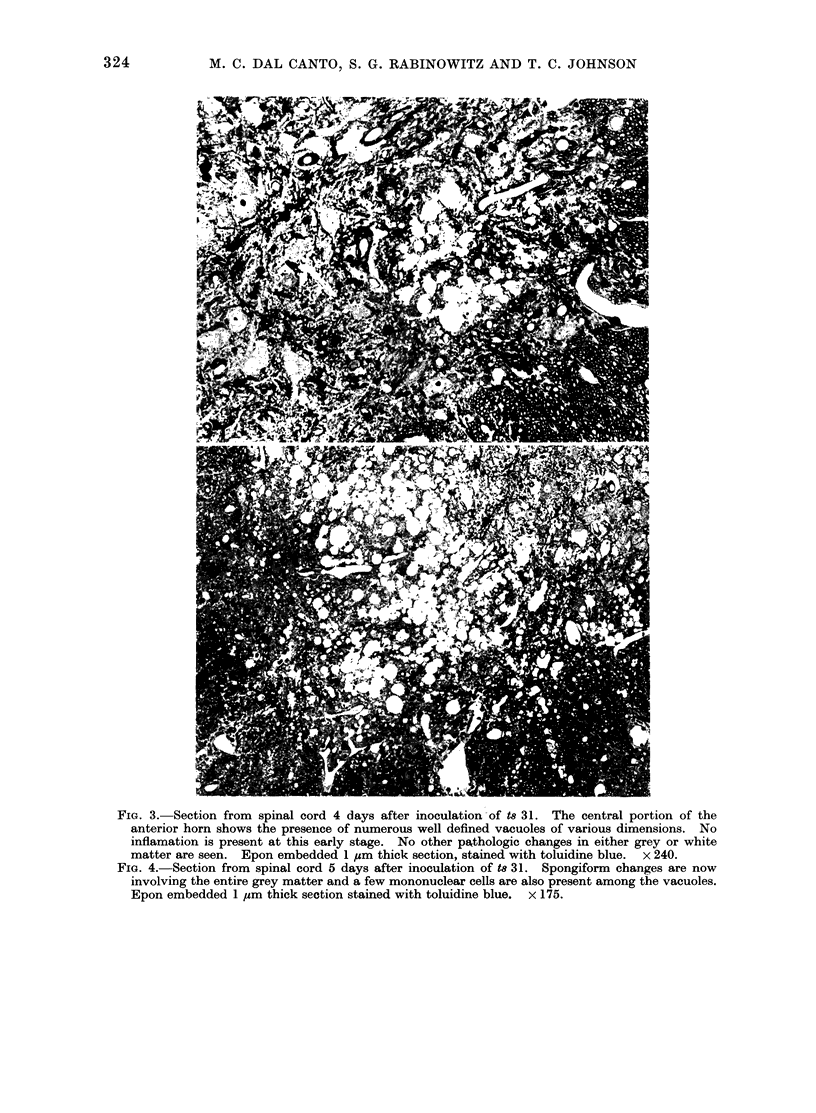

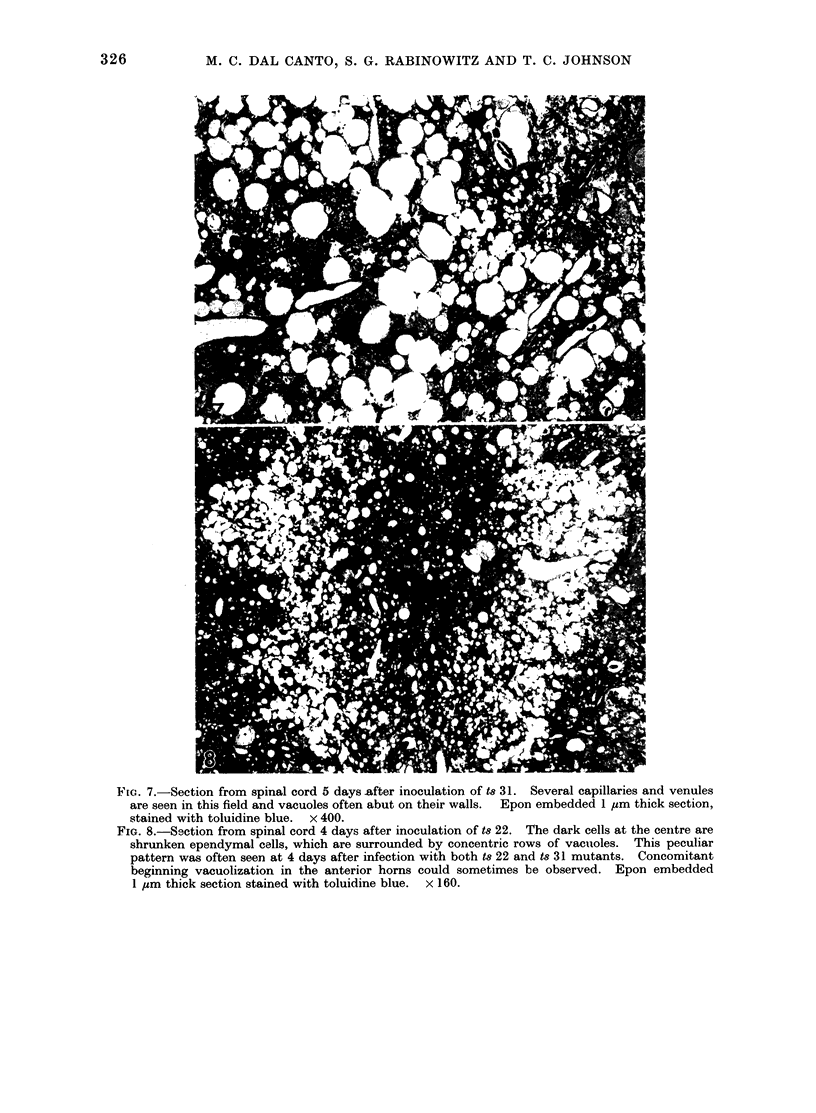




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleu F. P., Katzman R., Terry R. D. Fine structure and electrolyte analyses of cerebral edema induced by alkyl tin intoxication. J Neuropathol Exp Neurol. 1963 Jul;22(3):403–413. doi: 10.1097/00005072-196307000-00003. [DOI] [PubMed] [Google Scholar]
- Andrews J. M., Gardner M. B. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol. 1974 Apr;33(2):285–307. doi: 10.1097/00005072-197404000-00007. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Fenner F. Conditional lethal mutants of animal viruses. Curr Top Microbiol Immunol. 1969;48:1–28. doi: 10.1007/978-3-642-46163-7_1. [DOI] [PubMed] [Google Scholar]
- Fields B. N. Genetic manipulation of reovirus--a model for modification of disease. N Engl J Med. 1972 Nov 16;287(20):1026–1033. doi: 10.1056/NEJM197211162872007. [DOI] [PubMed] [Google Scholar]
- Gharpure M. A., Wright P. F., Chanock R. M. Temperature-sensitive mutants of respiratory syncytial virus. J Virol. 1969 Apr;3(4):414–421. doi: 10.1128/jvi.3.4.414-421.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Terry R. D., Weiss M. Electron microscopic study in two cases of Jakob-Creutzfeldt disease. J Neuropathol Exp Neurol. 1965 Oct;24(4):575–598. doi: 10.1097/00005072-196510000-00003. [DOI] [PubMed] [Google Scholar]
- Kimbrough R. D., Gaines T. B. Hexachlorophene effects on the rat brain: study of high doses by light and electron microscopy. Arch Environ Health. 1971 Aug;23(2):114–118. doi: 10.1080/00039896.1971.10665966. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Earle K. M., Gibbs C. J., Jr, Gajdusek D. C. Experimentak kuru encephalopathy in chimpanzees and spider monkeysElectron microscopic studies. J Neuropathol Exp Neurol. 1969 Jul;28(3):353–370. doi: 10.1097/00005072-196907000-00001. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Gajdusek D. C., Gibbs C. J., Jr Subacute spongiform virus encephalopathies. Scrapie, Kuru and Creutzfeldt-Jakob disease: a review. Am J Pathol. 1972 Sep;68(3):626–652. [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W., Schochet S. S. Electron microscopic observations on experimental spongy degeneration of the cerebellar white matter. J Neuropathol Exp Neurol. 1968 Apr;27(2):210–220. doi: 10.1097/00005072-196804000-00003. [DOI] [PubMed] [Google Scholar]
- Mackenzie J. S. Isolation of temperature-sensitive mutants and the construction of a preliminary genetic map for influenza virus. J Gen Virol. 1970 Jan;6(1):63–75. doi: 10.1099/0022-1317-6-1-63. [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Harter D. H., Hsu K. C. Neuropathological and immunofluorescence studies of experimental vesicular stomatitis virus encephalitis in mice. J Neuropathol Exp Neurol. 1971 Apr;30(2):266–277. doi: 10.1097/00005072-197104000-00008. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B. Preliminary physiological characterization of temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Jul;8(1):56–61. doi: 10.1128/jvi.8.1.56-61.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Pringle C. R., Follett E. A. Defective particles in BHK cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Aug;8(2):154–160. doi: 10.1128/jvi.8.2.154-160.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Hirst G. K. Temperature-sensitive mutants of influenza A virus: isolation of mutants and preliminary observations on genetic recombination and complementation. Virology. 1968 May;35(1):41–49. doi: 10.1016/0042-6822(68)90303-6. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kikkawa Y. Status spongiosus of CNS and hepatic changes induced by cuprizone (biscyclohexanone oxalyldihydrazone). Am J Pathol. 1969 Feb;54(2):307–325. [PMC free article] [PubMed] [Google Scholar]
- Takayama N., Nakano M. Pathogenicity of an attenuated, temperature-sensitive mutant of western equine encephalitis virus induced by a chemical mutagen. Infect Immun. 1975 Oct;12(4):858–865. doi: 10.1128/iai.12.4.858-865.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Sambrook J. F., Bellett A. J. Semliki forest virus temperature-sensitive mutants: isolation and characterization. Virology. 1969 Jul;38(3):427–439. doi: 10.1016/0042-6822(69)90155-x. [DOI] [PubMed] [Google Scholar]
- Zlotnik I., Grant D. P. The occurrence of vacuolated neurons in the brains of hamsters affected with subacute sclerosing encephalitis following measles or Langat virus infection. Br J Exp Pathol. 1975 Feb;56(1):72–76. [PMC free article] [PubMed] [Google Scholar]
- ter Meulen V., Koprowski H., Iwasaki Y., Käckell Y. M., Müller D. Fusion of cultured multiple-sclerosis brain cells with indicator cells: presence of nucleocapsids and virions and isolation of parainfluenza-type virus. Lancet. 1972 Jul 1;2(7766):1–5. doi: 10.1016/s0140-6736(72)91273-1. [DOI] [PubMed] [Google Scholar]