Abstract
While all three coactivators ARA70, steroid receptor coactivator 1, and RAC3/ACTR can enhance androgen receptor (AR) transcriptional activity at 1 nM dihydrotestosterone, we here demonstrate that only ARA70 can induce AR transcriptional activity >30-fold in the presence of 10 nM 17β-estradiol (E2), but not diethylstilbestrol. The significance of this newly described E2-induced AR transcriptional activity in DU145 human prostate cancer cells was further strengthened by finding patients with Reifenstein partial-androgen-insensitive syndrome that fail in the E2-AR-ARA70 pathway. Together, our data suggest, for the first time, testosterone/dihydrotestosterone may not be the only ligands for the AR. E2 represents another important natural ligand for AR that may play an essential role for the AR function and the development of the male reproductive system.
Keywords: androgen receptor coactivator, ARA70, SRC-1, RAC3/ACTR
The androgen receptor (AR) belongs to the steroid receptor superfamily that function primarily as transcription factors to regulate the expression of target genes by binding to specific hormone-responsive elements. These steroid receptors may exist within the target cells in a nonactivated state (1). After binding of the ligand, the activated receptors may interact with the hormone-responsive element, and the complex communicates with the transcriptional apparatus of the cell to induce or repress the expression of their target genes. It appears likely that coactivators or corepressors may be involved in this transactivation process and may function as a bridge between the receptor and the basal transcriptional factor complex (2, 3). Indeed, several coactivators or corepressors have been identified that may act as mediators to regulate the transactivation of the steroid receptors (4–12). As reported previously, we have successfully used the C-terminal domain of AR as bait to isolate the first AR coactivator, ARA70, that can enhance AR transcriptional activity an additional 6- to 10-fold in human prostate cancer cells (12).
Prostate cancer has become the most frequently diagnosed neoplasm in the United States and the second leading cause of cancer-related death in American men (13). So far, the only effective treatment for metastatic prostate cancer is androgen ablation by either surgical (orchiectomy) or chemical castration combined with antiandrogens. The rate of response to androgen ablation is high (≈80%), but the median duration of response is only 18–36 months (14). Several studies have postulated that changing the steroid specificity to activate mutant AR is one of the possible reasons (15, 16). Also, some data suggested other steroid hormones can activate AR transcriptional activity (17), but the real molecular mechanism remains unclear. Moreover, one potential explanation for abnormal growth of prostate cells in prostate cancer may be attributed to an early estrogen imprinting effect, and that estrogen has been suggested to play some important roles in the induction of spontaneous benign prostatic hyperplasia (18). The detailed mechanisms, however, remain to be elucidated. Although the 17β-estradiol (E2) binding to AR was shown by relative binding affinity to androgen, it is not clear whether E2 can therefore activate AR target genes and that any cellular factor(s) would contribute to this event. Thus, it becomes of interest to further investigate the effects of E2 on the transcriptional activity of wild type and mutant ARs in the presence and absence of coactivators.
Recently, we have successfully isolate the first relatively specific AR coactivator, ARA70 (12). In the current report, we present evidence to demonstrate that E2, but not diethylstilbestrol (DES) or other estrogens, can activate androgen target genes via its interaction with the AR-ARA70 complex. The results also argue strongly that in addition to the AR itself, the presence of ARA70 is an important factor for maximal induction of E2-mediated AR transcriptional activity. Together, our data suggest the specificity of estrogen vs. androgen may be conferred via the proper interaction of AR and ARA70.
MATERIALS AND METHODS
Chemicals and Materials.
The special purified 17β-E2 without androgen contamination was from Steroids, Inc. (Wilton, NH; with purity certification). Two other batches of highly purified 17β-E2 (purity > 99.8%) were from Sigma, and Steroids, Inc. All three 17β-E2 generated similar and reproducible results in our transfection assay. 17α-E2, DES, tamoxifen (Tam), and dexamethasone (Dex) were also from Sigma. ICI 182,780 (ICI) was kindly provided by A. Wakeling (Zeneca, Wilmington, DE).
Yeast and Mammalian Two-Hybrid Assay.
In the interaction growth assay, the AR bait and ARA70 cotransformed yeast Y190 cells were selected on plates with 25 mM 3-aminotriazole and a serial concentration of different hormones, but without histidine, leucine, or tryptophan (12). The toxicity of different hormones, dihydrotestosterone (DHT), Dex, 17α-E2, 17β-E2, and DES (from 10−9–10−5 M), to the yeast growth has been tested with general nutrition supply, and the growth variances are within 20%. The mammalian two-hybrid system used in our test system mainly follows the protocol of CLONTECH, with some modifications. To obtain better expression, the GAL4 DBD (amino acids 1–147) was fused to pSG5 that was driven by simian virus 40 promoter, and named GAL0. The hinge and ligand binding domain of wild-type AR and mutant ARe708k were then inserted into GAL0, respectively. Similarly, the VP16 activation domain was fused to pCMX, which was driven by cytomegalovirus promoter, and named pCMX-VP16 (provided by R. M. Evans, The Salk Institute for Biological Studies, La Jolla, CA). This pCMX-VP16 was then used to construct fusion of ARA70.
Transient Transfection and Chloramphenical Acetyltransferase (CAT) Assay.
In each transfection, 3.5 μg of mouse mammary tumor virus (MMTV)-CAT was used as reporter. One and one-half micrograms of hAR with or without 4.5 μg ARA70 or other cofactors were transfected into DU145 cells. Relative CAT activity was calculated by PhosphorImager quantification. β-Galactosidase activity was used to normalize transfection efficiency and to indicate cell viability under different concentrations of ligand treatment.
Ligand Binding Assay.
Binding of [3H]E2 (DuPont/NEN) was measured by the hydroxyapatite filter method (19). The result was expressed as a percentage of the label bound in the control tube (4,000 cpm) without additional unlabeled steroid. The experiment was repeated three times to assure steroid specificity.
RESULTS
Effects of Estrogens on the Interaction of Wild-Type AR and ARA70 in Yeast and Human Prostate Cells.
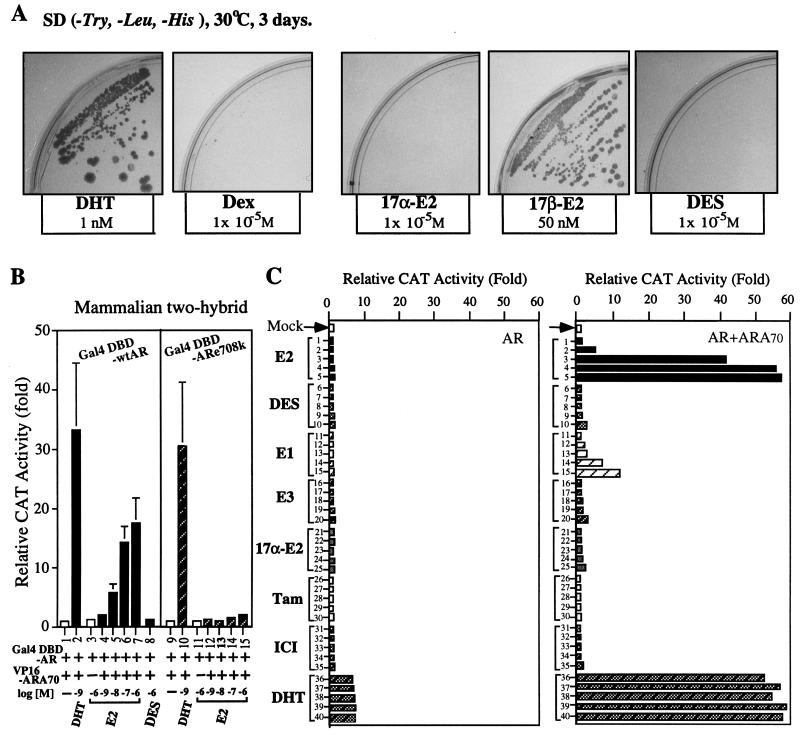
Using a yeast two-hybrid system under a serially diluted concentration of different hormones, we found that 50 nM E2 could induce an interaction between ARA70 and GAL4DBD-AR, but DES (a more potent, nonsteroidal estrogen) was unable to promote this interaction even at pharmacological concentrations (10−6–10−5 M, Fig. 1A shows the results of 10−5 M). Other nonandrogenic steroids such as Dex and 17α-E2 also did not induce any significant interaction (Fig. 1A). The specific dose-dependent E2 induced AR-ARA70 interaction (starting at physiological concentration 1 nM E2) was also demonstrated in the mammalian two-hybrid assay (Fig. 1B, lanes 4–7). Together, these data indicate that the E2-mediated physical association between AR and ARA70 exists both in yeast and mammalian cells.
Figure 1.
The interaction of ARA70 with AR in yeast and mammalian two-hybrid assay and the effect of ARA70 on the E2-mediated AR transcriptional activity. (A) Effects of DHT, Dex, 17β-E2, 17α-E2, and DES on the interaction of ARA70 with wild-type AR by plate nutritional selection in the yeast two-hybrid interaction system. The colonies formed on plates with 1 nM DHT, or 50 nM 17β-E2 but not on 10−5 M Dex, 17α-E2, or DES. Data were reproducible from two independent transformations. (B) Characterization of E2-mediated interaction between ARA70 and wild-type AR or mutant ARe708k by the mammalian two-hybrid assay. (C) Effects of E2, DES, estrone, estriol, 17α-E2, Tam, ICI, and DHT on the transcriptional activity of AR in the presence or absence of ARA70 in DU145 cells. After transfection, the cells were treated with serial concentrations of E2, DES, estrone, estriol, 17α-E2, Tam, ICI, and DHT (10−10 M: lanes 1, 6, 11, 16, 21, 26, 31, 36; 10−9 M: lanes 2, 7, 12, 17, 22, 27, 32, 37; 10−8 M: lanes 3, 8, 13, 18, 23, 28, 33, 38; 10−7 M: lanes 4, 9, 14, 19, 24, 29, 34, 39; 10−6 M: lanes 5, 10, 15, 20, 25, 30, 35, 40). The DHT treatments were taken as the positive control. Data represent an average of three independent experiments. The variance is ±15%.
Effects of E2 on the AR Transcriptional Activity in the Presence of ARA70.
Using DU145 cells, our MMTV-CAT assay data further showed that 1 nM E2 can start to induce transcriptional activity of AR (reaching >30-fold increase at 10 nM E2) only in the presence of ARA70 (Fig. 1C). DES and other estrogens/antiestrogens, such as estrone, 17α-E2, estriol, Tam, and ICI, showed very little induced activity, even at pharmacological concentration of 10−6M (Fig. 1C). A similar induction pattern mediated by E2 also occurred when we replaced MMTV-CAT with another androgen target gene, prostate-specific antigen-CAT (data not shown). As the contamination of androgen in the E2 used in our assay is not an issue (see Materials and Methods), these data strongly suggest that ARA70 may represent an important cofactor for the E2-mediated AR transcriptional activity.
The E2-Mediated Induction of AR Transcriptional Activity in the Presence of ARA70 Is Not Via Estrogen Receptor (ER), Progesterone Receptor (PR), or Glucocorticoid Receptor (GR).
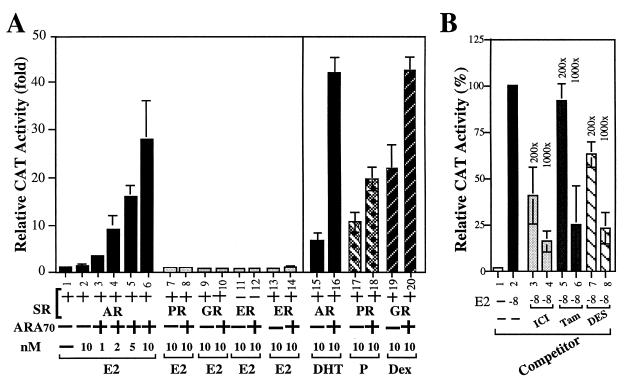
It is well documented that AR, GR, and PR could recognize the same consensus sequence. To rule out the possibility that E2-induced MMTV-CAT activity in the presence of ARA70 was mediated by other steroid receptors, such as PR, GR, or ER, we replaced AR with these receptors in our MMTV-CAT assay. As shown in Fig. 2A, our data demonstrate that only AR, but not ER, PR, or GR, can significantly induce MMTV-CAT activity in the presence of 1–10 nM E2 and ARA70 (Fig. 2, lanes 1–14). As there is no estrogen response element in MMTV promoter-CAT reporter, E2 cannot activate ER activity. The inclusion of ER in this experiment is to prove the E2-AR-ARA70 pathway is not through E2-ER. Again, our data also indicate that ARA70 only slightly enhances the transactivation of PR and GR in this assay (Fig. 2, lanes 17–20). Thus, ARA70 is a relatively specific coactivator for AR, which is consistent with our previous report (12).
Figure 2.
MMTV-CAT reporter was not activated through PR, GR, and ER in the present of ARA70 and 17β-E2. DU145 cells were cotransfected with 3.5 μg MMTV-CAT and 1.5 μg pSG5SR in the presence or absence of ARA70 under the same 10 nM E2 treatment (lanes 6–14). (A) The effect of ARA70 on other steroid receptors with the same reporter gene. The cells were transfected with AR/MMTV-CAT, GR/MMTV-CAT, PR/MMTV-CAT, in the presence of 10 nM DHT, P, or Dex, respectively (lanes 15–20). (B) DES, ICI, and Tam can inhibit the E2-mediated induction of AR transcriptional activity in the presence of ARA70. The induction of AR-ARA70 transcriptional activity by 10 nM E2 was counted as 100% (lane 2). The levels of increased inhibition relative to the concentration of E2 were shown as the following: ICI (lanes 3 and 4: 200-fold and 1,000-fold); Tam (lanes 5 and 6: 200-fold and 1,000-fold); DES (lanes 7 and 8: 200-fold and 1,000-fold).
As AR or ER could also be activated by phosphorylation (20, 21), it is possible that E2 may bind and activate ER, which somehow triggers a kinase system to phosphorylate and to activate the AR in our system. However, two factors argue strongly against this hypothesis. (i) The E2 effect on the association of AR and ARA70 has been verified in the budding yeast Y190, Saccharomyces cerevisiae, which contains no endogenous ER (22). (ii) Other estrogenic compounds, including DES, a more potent estrogen than E2, can induce neither the association of AR and ARA70 in yeast nor the transcriptional activity of AR and ARA70 in DU145 cells (Fig. 1). Based on these two findings, we therefore believe that AR and ARA70, but not the ER, are the essential factors for E2-induced AR transcriptional activity.
While Fig. 1C suggests that DES, Tam, and ICI by themselves cannot induce any significant AR transcriptional activity at concentrations of 10−8–10−6 M, Fig. 2B shows that at 2 × 10−6–10−5 M (200- and 1,000-fold concentrations of 10−8M E2), DES, Tam, and ICI can repress E2-mediated induction of AR transcriptional activity. These data suggest that DES, Tam, and ICI may share some, but not all, E2 binding sites in the AR-ARA70 complex. A crystallography study of AR-ARA70 in the presence of E2 or DES should be able to answer this question.
E2 Binding of AR and ARA70.
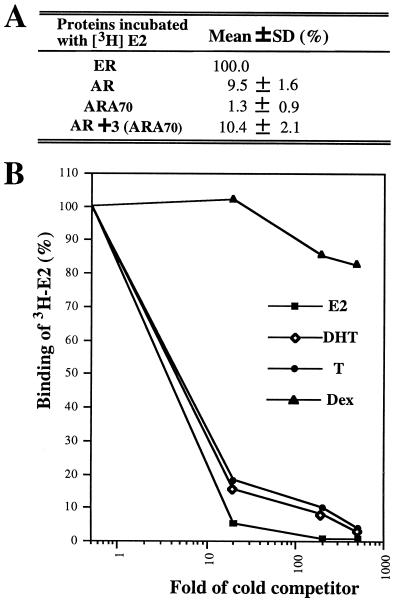
A cell-free in vitro transcription/translation system was used to test whether ARA70 can either bind to E2 or increase the AR binding to E2. As shown in Fig. 3A, [3H]E2 has 10% and 1% (using E2-ER as 100%) of the relative binding affinity to AR and ARA70, respectively. AR and ARA70 mixed at 1:3 ratio (the ratio for the optimal E2-induced AR activity) did not increase the affinity of E2 to AR.
Figure 3.
E2-Ligand binding of AR and ARA70. (A) In vitro synthesized AR, ARA70, and ER were quantitated by [35S]methionine labeling. Equal molar concentrations of ER and AR were used for the [3H]E2 ligand binding assay. Three-fold molar ARA70 was incubated with AR on ice for 1 hr before adding 50 nM [3H]E2. Two hundred-fold unlabeled E2 was used as a competitor to determine the specific binding. (B) E2-specific binding of full-length AR. AR was transcribed and translated in a rabbit reticulocyte lysate system. Aliquots of the lysate were then incubated with 50 nM [3H]E2 (87 Ci/mmol; 1 Ci = 37 GBq) in the presence or absence of 20-fold, 200-fold, and 500-fold unlabeled steroids. The final incubation volume was 100 μl. The values of duplicate assay tubes were within 10% of the average shown in the figure.
The competitive binding curve in Fig. 3B further showed that unlabeled E2, T, and DHT, but not Dex, can compete well with [3H]E2 binding to AR, indicating E2 specific binding between E2 and AR. The fact that unlabeled E2 can compete better than T and DHT with [3H]E2-AR binding suggested that E2-AR and DHT-AR may have different conformation. This hypothesis is further supported by results from the studies of the interaction between mutant AR and ARA70 (see Fig. 5).
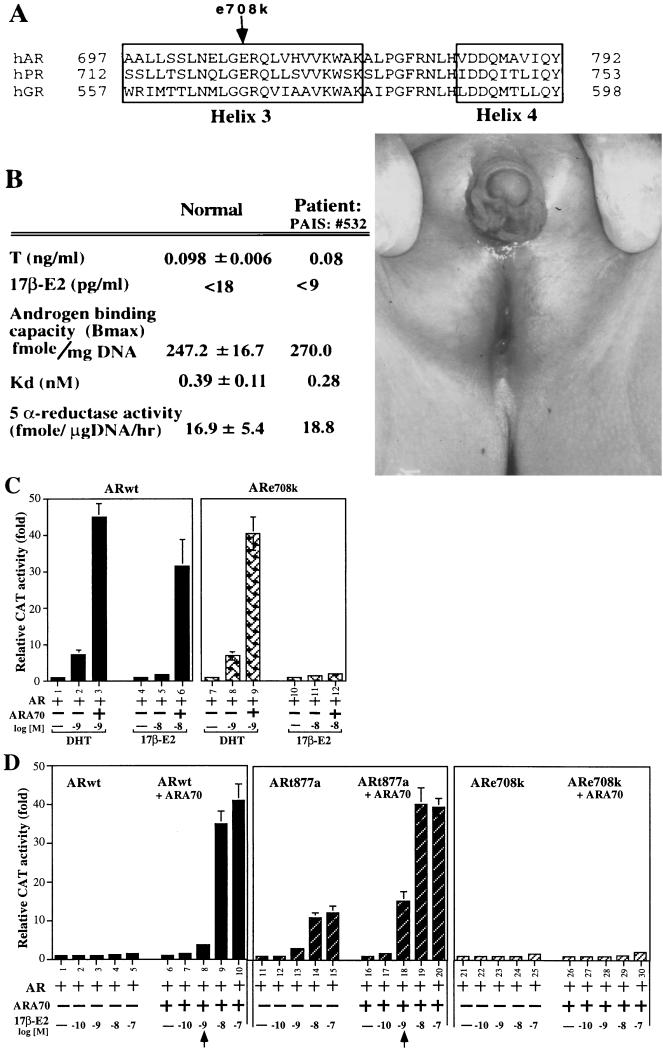
Figure 5.
The E2-mediated induction of AR transcriptional activity on mutant ARs. The ARe708k was from the partial-androgen-insensitive syndrome patient. The ARt877a was from LNCaP and prostate cancer patient. Fixed amount of AR and ARA70 were used in transfection. (A) A schematic representation of the helix 3 of AR, PR, and GR showing the location of mutant e708k. (B) The bioprofile of the partial-androgen-insensitive syndrome patient with mutant ARe708 k and the physical defect in the exterior reproductive system of the patient. (C) Effects of DHT and E2 on the transcriptional activity of wild-type AR, and ARe708k in the presence or absence of ARA70 in DU145 cells. (D) Effects of E2 on the transcriptional activity of wild-type AR, mutant ARt877a, and ARe708k in the presence or absence of ARA70.
Together, our ligand binding assay suggests that, while the E2-AR binding is specific, ARA70 by itself does not bind E2, or increase the affinity of E2 binding to AR. However, it is possible that the classic ligand binding assay could not detect the subtle increase of E2-AR binding that is required for E2-induced AR activity.
ARA70 Is the Effective Coactivator for E2-Mediated AR Transcriptional Activity.
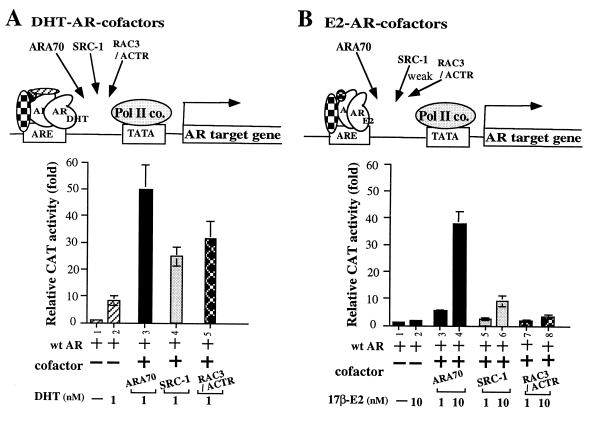
To investigate whether E2-AR-ARA70 forms a special complex with a distinct conformation to mediate E2-induced AR activity, we compared three coactivators, SRC-1 (4), RAC3/ACTR (10, 11), and ARA70 for their DHT- and E2-induced AR activity. As shown in Fig. 4A, all three coactivators can enhance AR transcriptional activity at 1 nM DHT. Although it has been speculated that SRC-1 and RAC3/ACTR are the coactivators for many steroid hormone receptors, this is the first evidence showing that SRC-1 and RAC3/ACTR do functionally enhance the transactivation of AR. While all three coactivators enhance DHT-AR transcriptional activity, only ARA70 can induce AR activity from the concentration of 1 nM E2 and shows >30-fold induction at 10 nM E2 (Fig. 4B) These data suggest that DHT-AR complex is sufficiently different from E2-AR that only ARA70 can confer the significant androgenic activity on E2.
Figure 4.
The comparison of three coactivators, ARA70, SRC-1, RAC3/ACTR on DHT- and E2- mediated AR transcriptional activity. Fixed amounts of AR and cofactors were used in transfections.
The E2-Mediated Induction of AR Transcriptional Activity on Mutant ARs in the Presence or Absence of ARA70.
To extend our E2-induced AR activity in DU145 cells in vivo, we first used the yeast mutagenesis system to screen for AR mutants that will not respond to E2-induced AR activity. An initial candidate with a mutation at amino acid 708 (glutamic acid to lysine, named ARe708k) was identified and preliminary data suggested that ARe708k maintained DHT-induced AR activity but somehow lost E2-induced AR activity (23). Mammalian two-hybrid system also indicated that only DHT, but not E2, can promote the interaction of ARe708k and ARA70 (Fig. 1B). Based on recent publications about the crystal structure of liganded and unliganded steroid receptor ligand binding domains, the ligand binding cavity is speculated to be formed by parts of helix 3 (H3), H4, H6, H11, H12, and the S1/S2 hairpin (24, 25). Our data further suggest that H3 is essential for the formation of ligand binding cavity. The change of the charge from glutamic acid to lysine on residue 708 is likely to influence the ligand binding. A further crystallography study of AR in the presence of E2 or DHT should be able to define the subtle structure differences of wtAR and mutant ARe708k.
Suspecting that some patients with androgen response disorders may carry ARe708k, we identified one Reifenstein syndrome patient with partial androgen insensitivity, who does indeed carry the ARe708k mutation. As shown in Fig. 5, while this patient had abnormal male reproductive organs, he had normal testosterone and E2 concentrations. Primary cultures of genital skin fibroblasts from this patient also showed normal androgen binding capacity, Kd for DHT to AR, and 5 α-reductase activity. Surprisingly, when we replaced the patient’s mutant ARe708k with wild-type AR in the E2-AR-ARA70 MMTV-CAT assay, we found that ARe708k has only slightly reduced DHT-mediated AR activity in the presence of ARA70 (Fig. 5C lane 3 vs. 13) or absence of ARA70 (Fig. 5C lane 2 vs. 12). The only significant difference in this patient’s androgen activity, as compared with normal, is that his mutated ARe708k cannot enhance E2-mediated AR activity in the presence of ARA70 (Fig. 5C lane 6 vs. 16; Fig. 5D lanes 9 and 10 vs. 29 and 30). The consequence of losing this E2-AR-ARA70 pathway while maintaining the DHT-AR and DHT-AR-ARA70 pathways may likely be one of the explanations for the Reifenstein syndrome with partially abnormal male reproductive organs (Fig. 5B). The inclusion of a mutant AR (ARt877a, threonine to alanine) found in many prostate tumors is not only to demonstrate that E2-AR-ARA70 can also function at physiological concentrations (1 nM E2) in prostate tumor but also to test the widely accepted hypothesis that a single amino acid mutation of the AR can allow E2 to induce AR activity (16, 17). As shown in Fig. 5D, while ARt877a mutant may increase the potency of E2 (lanes 4 vs. 14 and 8 vs. 18), a mutated ARt877a alone exhibits a relatively small induction of its AR transcriptional activity by E2, and ARA70 is still required to maximize E2-induced AR activity. Furthermore, using transient transfection assay, we have investigated the E2-AR-ARA70 transcriptional activity in PC3 and CHO cells that express endogenous ARA70. Because these cells may have higher expression of endogenous ARA70, the cotransfection of ARA70 cannot significantly induce the activity, but we are able to demonstrate that antisense ARA70 can block 35–45% of E2-mediated AR activity (data not shown). Together, these data suggested that ARA70 plays important roles in the proper or maximal E2-mediated AR transcriptional activity.
DISCUSSION
While mutated ARs become widely accepted as the explanation for why E2 may induce prostate-specific antigen in prostate cancer cells (16, 17), we now present evidence demonstrating that E2 can activate AR target genes, such as MMTV-long terminal repeat or prostate-specific antigen, in the presence of wild-type AR and ARA70 at 1:3 ratio. These data, along with evidence from the comparison of three cofactors (Fig. 4), suggest that ARA70 is a key factor for the E2- and androgen-mediated induction of androgen target genes in prostate. Therefore, a drug designed to block the interaction of AR and ARA70 could have significant therapeutic and perhaps preventative value.
Even after several decades, surgical or medical castration combined with the administration of antiandrogens remains as the major treatment for disseminated prostate cancer. Estrogens are still at times used to repress androgens, and currently provide a very favorable cost profile compared with any other means of androgen ablation. Many clinical trials have suggested that E2 would be less effective than DES (26, 27) when used to treat prostate cancer. Our findings that E2, but not DES, can activate androgen-target genes in the prostate (Fig. 1) may, therefore, provide one explanation for this observation. Also, the fact that DES may block E2-mediated AR activity may attribute a new antiandrogenic function to DES (Fig. 2B). Our observation that E2 and DES can have different functions (only E2 can activate androgen-target genes), may also help elucidate the complicated molecular mechanisms of estrogen/antiestrogen interactions and effects.
Other reports also suggest that estrogens may have imprinting effects on prostate growth (18) and can induce prostatic dysplasia and tumors in Noble rats (28). Whether all of these estrogenic effects are mediated through the ER or some are due to estrogen’s interaction with the AR and ARA70 will be an interesting question to investigate.
It is well documented that the transactivation of target genes by steroid receptor is dependent on the context of both the target promoter and the transfected cell (29, 30). Furthermore, transcriptional interference/squelching has been observed between the activation functions of the various steroid receptors (31, 32). These observations provide initial evidence for the existence of transcriptional mediator(s)/cofactor(s). Consequently, several cofactors were cloned by virtue of yeast two-hybrid or far-Western screening (4–12). Recently, Katzenellenbogen and O’Malley proposed a tripartite system (ligand-receptor-cofactor) to explain the molecular interactions of steroid receptors that may define the potency and biological character of steroid hormones (33). They postulated that mutant ER was much less effective than wild-type ER in stimulating transcription, and that different responses to estrogen or antiestrogens may not be necessarily due to a change in ER expression level or the binding affinity to ligands (33, 34). Rather, the data support the hypothesis that the conformation of estrogen-ER and antiestrogen-ER complexes may be different and that the potential for interaction with receptor cofactors may also be different (35). Therefore, they proposed that ligand-receptor interactions alone may not be able to control the response, and the interaction between ligand-receptor complexes and cofactors may be essential for steroid hormone function and selectivity. Our finding that E2 can activate androgen target genes in the presence of AR and ARA70, therefore, may represent the direct evidence to support the “tripartite receptor system” concept and indicates that the receptor specificity (E2 binding to AR) and biological diversity (E2 activates AR target genes) of the steroid hormone family can be conferred at the level of cofactors.
The discovery of this new pathway from E2 to AR-ARA70 for the activation of androgen target genes in human prostate may have some important implications. (i) It suggests that testosterone/DHT may not be the only ligands for AR, and E2 can be another natural ligand for AR, which plays an important role in the development of the male reproductive system. (ii) Our observation that E2 and DES can have different functions (only E2 can activate androgen-target genes) may help to explain why E2 is less effective than DES, when used to treat prostate cancer; this may have many clinically important ramifications when estrogens are used as therapy for androgen-related disorders. Finally, as several estrogenic compounds may play a role in the disruption of normal endocrine functions in humans and other animals (36), it will be interesting to know whether any environmental pollutants-estrogenic compounds also have some androgenic activity that may contribute to the disruption of the endocrine system.
In summary, the new E2-AR-ARA70 pathway found in human prostate cancer cells suggests that a special coactivator ARA70 may be able to modulate the sex hormone specificity. Moreover, E2 may represent an essential ligand of AR that plays an important role in the development and/or functioning of the male reproductive system. Further studies of this E2-AR-ARA70 pathway may therefore allow us to develop new hormonal therapies for the treatment of prostate cancer and other androgen-related disorders.
Acknowledgments
We thank Dr. J. Pink, H. Ting and J. Hsu for technical support. SRC-1 and RAC3/ACTR are gifts from Drs. B. W. O’Malley and J. D. Chen. This work was supported by National Institutes of Health Grants CA55639, CA68518, and CA71570.
ABBREVIATIONS
- AR
androgen receptor, ER, estrogen receptor
- GR
glucocorticoid receptor
- PR
progesterone receptor
- DES
dihydrostilbestrol
- DHT
dihydrotestosterone
- CAT
chloramphenical acetyltransferase
- E2
17β-estradiol
- MMTV
mouse mammary tumor virus
- Dex
dexamethasone
- Tam
tamoxifen
- ICI
ICI 182,780
References
- 1.Pratt W B, Welsh M J. Semin Cell Biol. 1994;5:83–93. doi: 10.1006/scel.1994.1012. [DOI] [PubMed] [Google Scholar]
- 2.Tsai M J, O’Malley B W. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 3.Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L. Cell. 1994;79:107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 4.Onate S A, Tsai S Y, Tsai M J, O’Malley B W. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 5.Horlein A J, Naar A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass C K, et al. Nature (London) 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen J D, Evans R M. Nature (London) 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 7.Cavailles V, Dauvois S, L’Horset F, Lopez G, Hoare S, Kushner P J, Parker M G. EMBO J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, et al. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 9.vom Baur E, Zechel C, Heery D, Heine M J, Garnier J M, Vivat V, Le Douarin B, Gronemeyer H, Chambon P, Losson R. EMBO J. 1996;15:110–124. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Gomes P J, Chen J D. Proc Natl Acad Sci USA. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh S, Chang C. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker S L, Tong T, Bolden S, Wingo P A. Ca Cancer J Clin. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Crawford E D. Br J Urol. 1992;70:33–38. doi: 10.1111/j.1464-410x.1992.tb15865.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilding G, Chen M, Gelmann E P. Prostate. 1989;14:103–115. doi: 10.1002/pros.2990140204. [DOI] [PubMed] [Google Scholar]
- 16.Veldscholte J, Voorhorst-Ogink M M, Bolt-de Vries J, van Rooij H C, Trapman J, Mulder E. Biochim Biophys Acta. 1990;1052:187–194. doi: 10.1016/0167-4889(90)90075-o. [DOI] [PubMed] [Google Scholar]
- 17.Taplin M E, Bubley G J, Shuster T D, Frantz M E, Spooner A E, Ogata G K, Keer K N, Balk S P. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 18.Rajfer J, Coffey D S. Urology. 1978;16:186–190. [PubMed] [Google Scholar]
- 19.Chang C, Kokontis J, Liao S. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- 20.Nazareth L V, Weigel N L. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 21.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotch Y, Nishida E, Kawashima H, et al. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 22.Metzger D, White J H, Chambon P. Nature (London) 1988;334:31–36. doi: 10.1038/334031a0. [DOI] [PubMed] [Google Scholar]
- 23.Wang C. Ph.D. thesis. Madison: Univ. of Wisconsin; 1997. [Google Scholar]
- 24.Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D. Nature (London) 1995;375:377–382. doi: 10.1038/375377a0. [DOI] [PubMed] [Google Scholar]
- 25.Brzozowski A M, Pike A C W, Dauter Z, Hubbard R E, Bonn T, Engstrom O, Dhman L, Greene G L, Gustafsson J-A, Carlquist M. Nature (London) 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 26.Bissada N K, Kaczmarek A T. J Urol. 1995;153:1944–1945. [PubMed] [Google Scholar]
- 27.Vogelzang N J, Kennealey G T. Cancer (Philadelphia) 1992;70:966–976. [PubMed] [Google Scholar]
- 28.Ofner P, Bosland M C, Vena R L. Toxicol Appl Pharmacol. 1992;112:300–309. doi: 10.1016/0041-008x(92)90200-c. [DOI] [PubMed] [Google Scholar]
- 29.Berry M, Metzger D, Chambon P. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagpal S, Saunders M, Kastner P, Durand B, Nakshatri H, Chambon P. Cell. 1992;70:1007–1019. doi: 10.1016/0092-8674(92)90250-g. [DOI] [PubMed] [Google Scholar]
- 31.Bocquel M T, Kumar V, Stricker C, Chambon P, Gronemeyer H. Nucleic Acids Res. 1989;17:2581–2595. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tasset D, Tora L, Fromental C, Scheer E, Chambon P. Cell. 1990;62:1177–1187. doi: 10.1016/0092-8674(90)90394-t. [DOI] [PubMed] [Google Scholar]
- 33.Katzenellenbogen J A, O’Malley B W, Katzenellenbogen B S. Mol Endocrinol. 1996;10:119–131. doi: 10.1210/mend.10.2.8825552. [DOI] [PubMed] [Google Scholar]
- 34.Pink J J, Jordan V C. Cancer Res. 1996;56:2321–2330. [PubMed] [Google Scholar]
- 35.Montano M M, Muller V, Trobaugh A, Katzenellenbogen B S. Mol Endocrinol. 1995;9:814–825. doi: 10.1210/mend.9.7.7476965. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser J. Science. 1996;272:1418. doi: 10.1126/science.272.5267.1418. [DOI] [PubMed] [Google Scholar]