Abstract
The infectivity of 16 strains of Klebsiella spp. and its modification by systemic and local ferric iron were tested in the skin of the guinea-pig. The in vivo proliferation of 11 strains was enhanced in varying degrees by Fe+++ (E + strains); 5 strains (Eo) were not enhanceable even by large doses of Fe+++. Of 10 strains examined in detail, 6 were E + and 4 were E0. Guinea-pig and human sera were consistently bacteriostatic for E + strains and bactericidal for Eo strains. Both Fe+++ and microbial iron-chelators abolished the bacteriostasis of E + strains but did not affect the lethal effect on Eo strains. Both effects were diminished by heating the sera to 56 degrees for 30 min and by the anticomplementary substance Liquoid; neither appeared to be due to specific antibody. Virulence, as measured in the skin and by intravenous injection, was roughly associated with degree of enhanceability by iron, the EO strains being among the least virulent. The volume of plasma exudate entering the skin during the first 5 h was sufficient to kill a large proportion of the infecting doses of Eo strains and to inhibit the growth of infecting doses of E + strains. Enhancement of the latter by Fe+++ is predominantly the result of inhibition of the non-specific bacteriostasis exerted by the extravascular plasma. Lesions by E + strains aged 4 h or more are insusceptible to systemic Fe+++ and only moderately susceptible to large doses of local Fe+++. The insusceptibility appears to be due to segregation of the infecting bacilli within exudate leucocytes. Klebsiella infections accordingly provide another example of an initial decisive period of action of the antibacterial defences-in this case non-specific and humoral-which cease to be locally effective after the first few hours. Besides enhancing lesions due to E + strains, systemic Fe+++ has an opposite, apparently anti-inflammatory action on klebsiella lesions, slightly decreasing their size. It was evident with all the strains tested, whether dead or alive, but not in E + lesions in circumstances when they were susceptible to enhancement by the Fe+++.
Full text
PDF


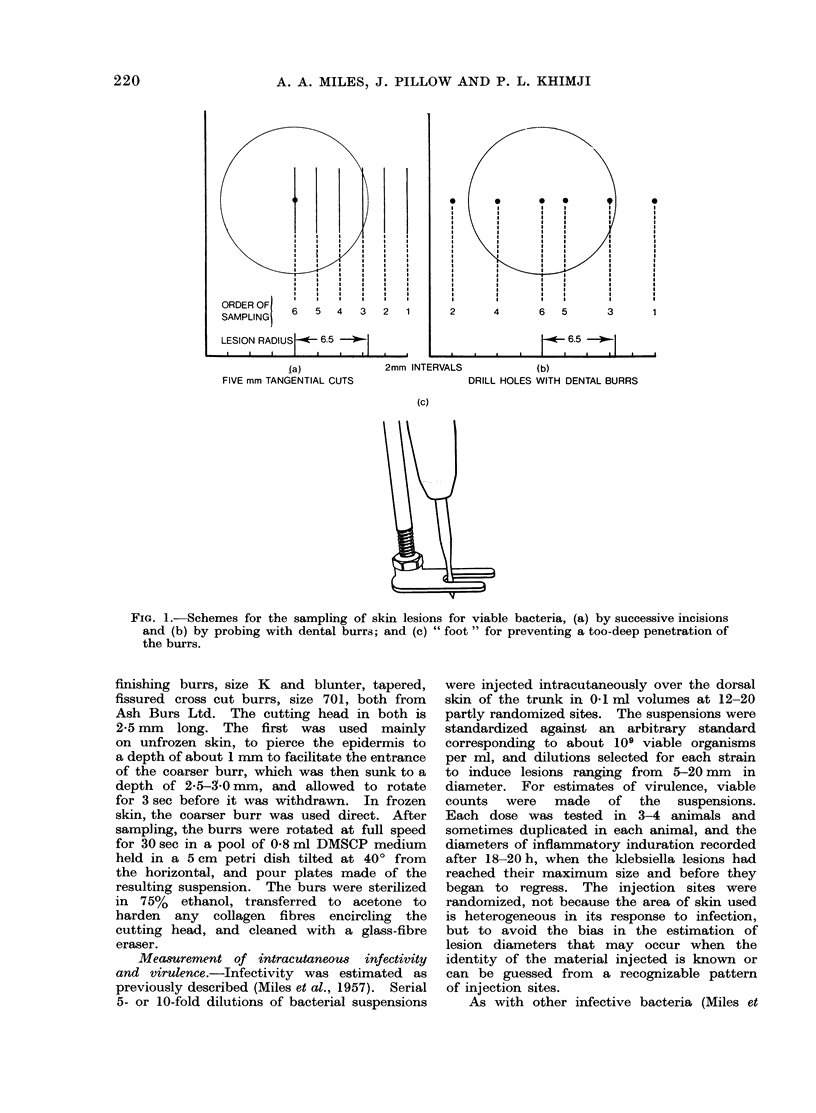
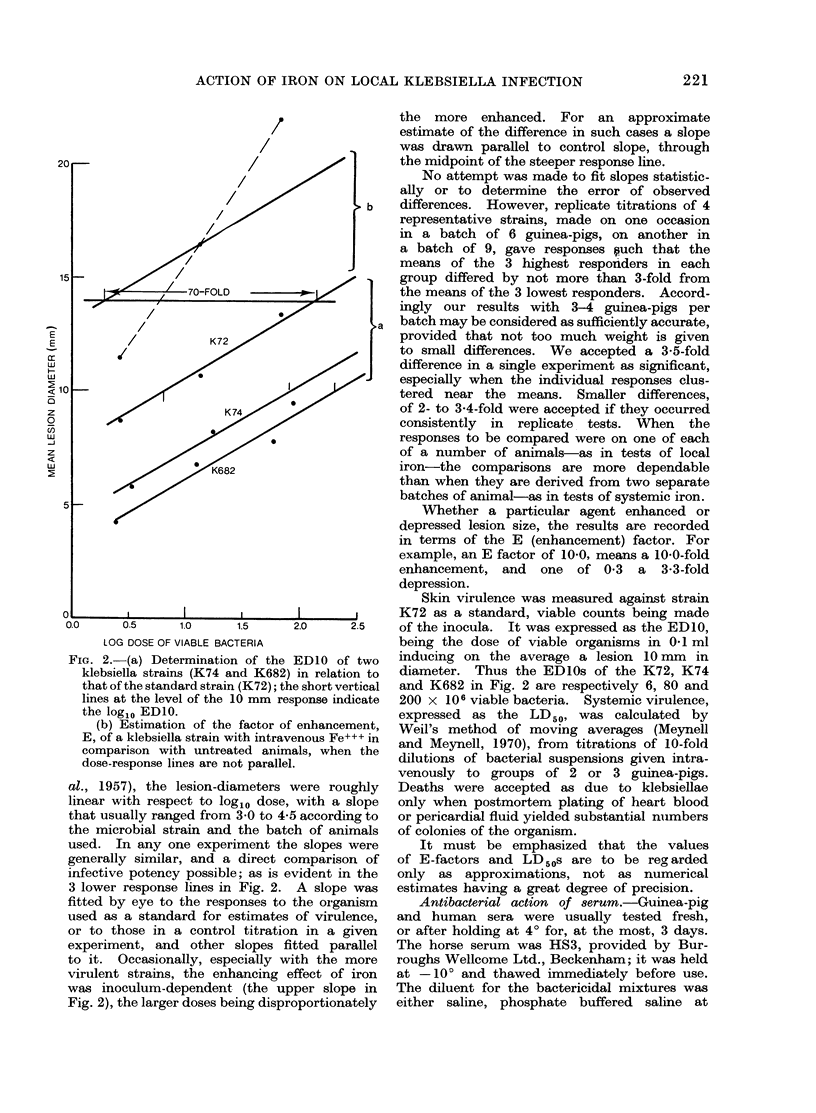
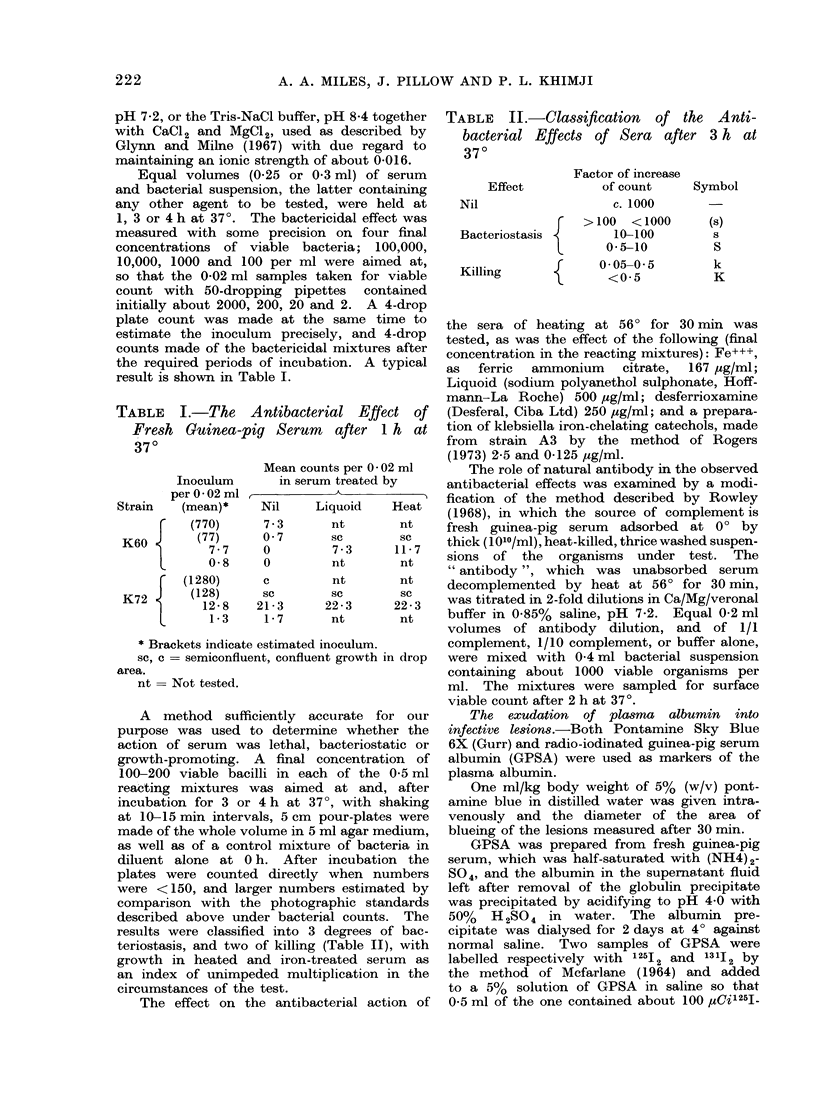

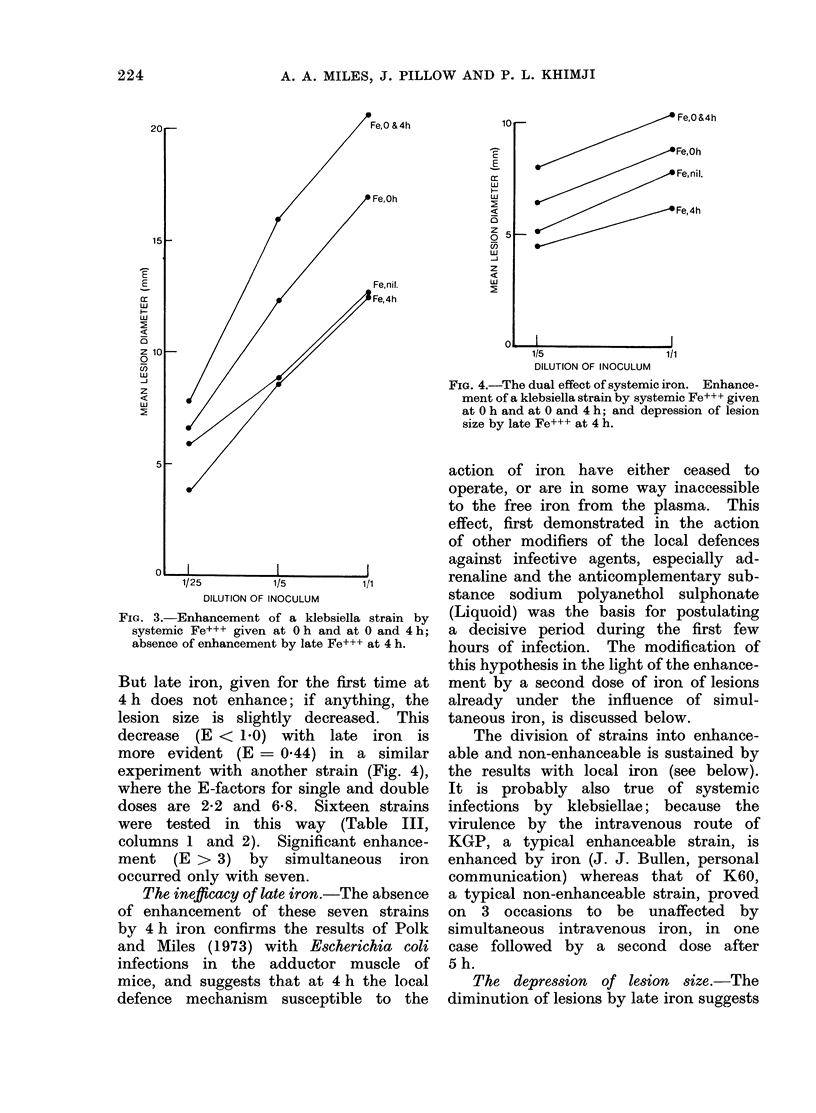







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANCILL R. J. The blood volume of the normal guinea-pig. J Physiol. 1956 Jun 28;132(3):469–475. doi: 10.1113/jphysiol.1956.sp005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Cushnie G. H., Rogers H. J. The abolition of the protective effect of Clostridium welchii type A antiserum by ferric iron. Immunology. 1967 Mar;12(3):303–312. [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. M., Bullen J. J. The effect of passage and iron on the virulence of Pseudomonas aeruginosa. J Clin Pathol. 1972 Jan;25(1):65–68. doi: 10.1136/jcp.25.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone G. P., Walton E. The effect of iron and haematin on the killing of staphylococci by rabbit polymorphs. Br J Exp Pathol. 1971 Oct;52(5):452–464. [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Milne C. M. A kinetic study of the bacteriolytic and bactericidal action of human serum. Immunology. 1967 Jun;12(6):639–653. [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Comparative bactericidal activities of blood serum and plasma serum. J Exp Med. 1960 Jul 1;112:15–22. doi: 10.1084/jem.112.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANOFF A., ZWEIFACH B. W. Inactivation of bacterial exotoxins and endotoxin by iron. In vitro studies. J Exp Med. 1960 Jul 1;112:23–34. doi: 10.1084/jem.112.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILES A. A., MILES E. M., BURKE J. The value and duration of defence reactions of the skin to the primary lodgement of bacteria. Br J Exp Pathol. 1957 Feb;38(1):79–96. [PMC free article] [PubMed] [Google Scholar]
- MILES A. A., MILES E. M. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol. 1952 Oct;118(2):228–257. doi: 10.1113/jphysiol.1952.sp004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masawe A. E., Muindi J. M., Swai G. B. Infections in iron deficiency and other types of anaemia in the tropics. Lancet. 1974 Aug 10;2(7876):314–317. doi: 10.1016/s0140-6736(74)91693-6. [DOI] [PubMed] [Google Scholar]
- McFarlane A. S., Koj A. Short-term measurement of catabolic rates using iodine-labeled plasma protein. J Clin Invest. 1970 Oct;49(10):1903–1911. doi: 10.1172/JCI106409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Polk H. C., Miles A. A. The decisive period in the primary infection of muscle by Escherichia coli. Br J Exp Pathol. 1973 Feb;54(1):99–109. [PMC free article] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative bacteria by lysozyme. Biochim Biophys Acta. 1956 Oct;22(1):189–191. doi: 10.1016/0006-3002(56)90240-2. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. Sensitivity of rough gram-negative bacteria to the bactericidal action of serum. J Bacteriol. 1968 May;95(5):1647–1650. doi: 10.1128/jb.95.5.1647-1650.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLAW A. C. The complement-dependent bacteriolytic activity of normal human serum. I. The effect of pH and ionic strength and the role of lysozyme. J Exp Med. 1962 Jun 1;115:1231–1249. doi: 10.1084/jem.115.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Role of iron in host-parasite interactions. J Infect Dis. 1971 Oct;124(4):401–410. doi: 10.1093/infdis/124.4.401. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Morley J. Measurement of rate of extravasation of plasma protein in inflammatory responses in guinea-pig skin using a continuous recording method. Br J Exp Pathol. 1974 Feb;55(1):1–12. [PMC free article] [PubMed] [Google Scholar]


