Abstract
The phenomenon of cell transformation by type 2 herpes simplex virus has been investigated. Primary hamster embryo fibroblasts were exposed to type 2 herpes virus under conditions which would restrict or inhibit the lytic events of virus-cell interaction. Cell lines were established by single-cell cloning. There was evidence of altered cell morphology with altered biological activity in terms of longevity and oncogenicity; there was, however, no evidence of virus specific antigen or incorporation of viral nucleic acid into the host cell genome. Virus specific antigen was only detected in the early passages of an uncloned transformed cell line. We are thus unable to confirm previous studies (vide supra) and are obliged to propose a "hit and run" model for in vitro cell transformation by type 2 herpes simplex virus.
Full text
PDF




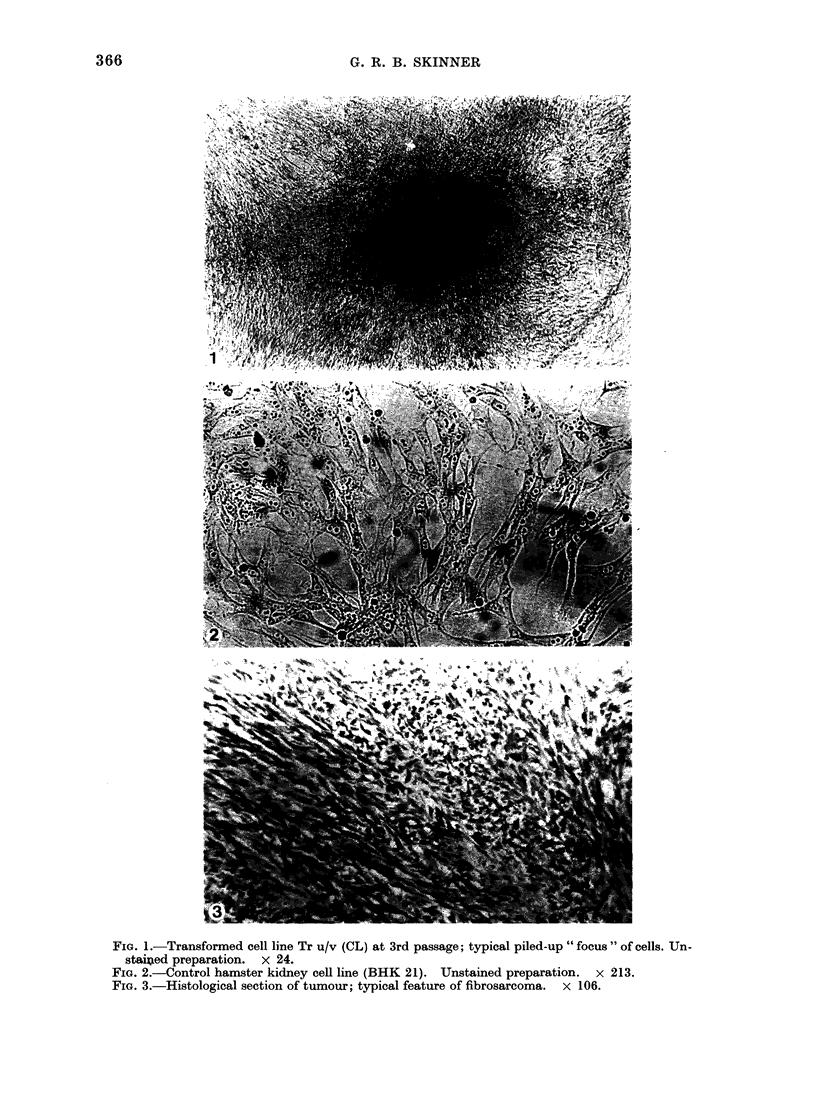
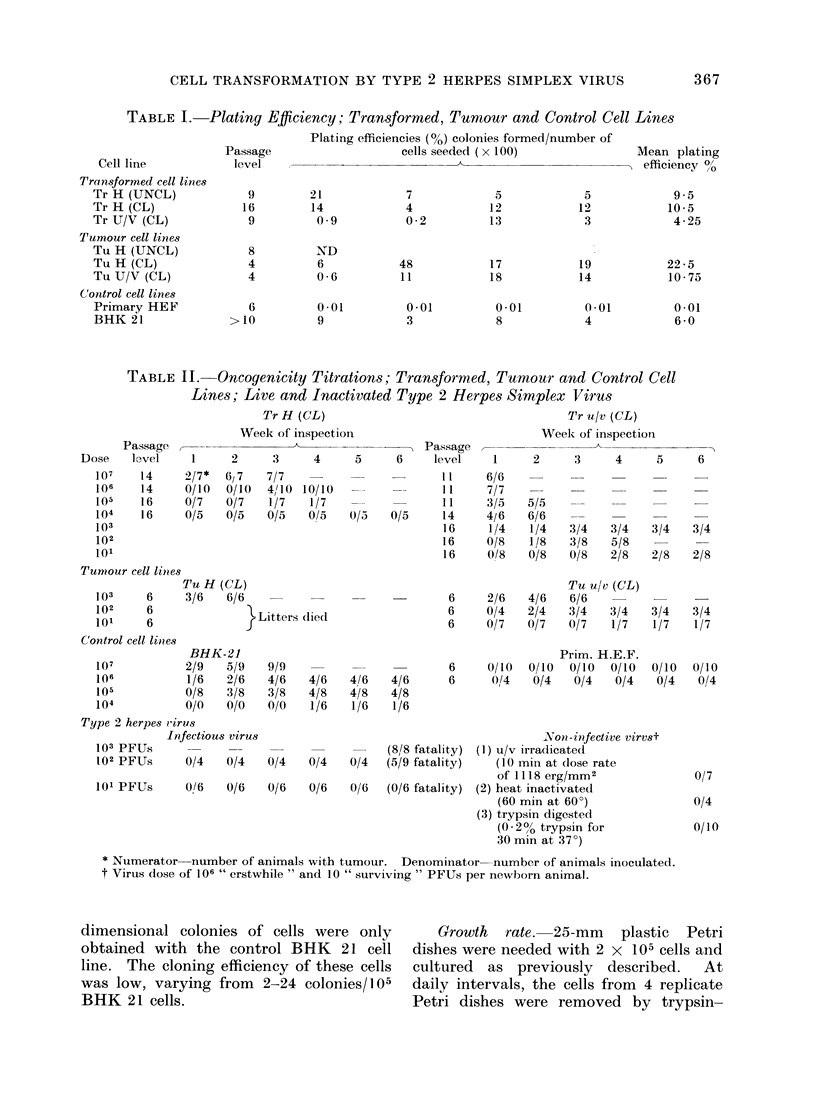







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. SV40 T antigen induction and transformation in human fibroblast cell strains. Virology. 1968 Oct;36(2):254–261. doi: 10.1016/0042-6822(68)90142-6. [DOI] [PubMed] [Google Scholar]
- Ablashi D. V., Loeb W. F., Valerio M. G., Adamson R. H., Armstrong G. R., Bennett D. G., Heine U. Malignant lymphoma with lymphocytic leukemia induced in owl monkeys by Herpesvirus saimiri. J Natl Cancer Inst. 1971 Oct;47(4):837–855. [PubMed] [Google Scholar]
- Adam E., Kaufman R. H., Melnick J. L., Levy A. H., Rawls W. E. Seroepidemiologic studies of herpesvirus type 2 and carcinoma of the cervix. IV. Dysplasia and carcinoma in situ. Am J Epidemiol. 1973 Aug;98(2):77–87. doi: 10.1093/oxfordjournals.aje.a121541. [DOI] [PubMed] [Google Scholar]
- Aurelian L., Strandberg J. D., Melendez L. V., Johnson L. A. Herpesvirus type 2 isolated from cervical tumor cells grown in tissue culture. Science. 1971 Nov 12;174(4010):704–707. doi: 10.1126/science.174.4010.704. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P., TURNER H. C., HUEBNER R. J. A SPECIFIC COMPLEMENT-FIXING ANTIGEN PRESENT IN SV40 TUMOR AND TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Dec;50:1148–1156. doi: 10.1073/pnas.50.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard W., Thornton H., Green M. Cells transformed by human Herpesvirus type 2 transcribe virus-specific RNA sequences shared by Herpesvirus types 1 and 2. Nat New Biol. 1973 Jun 27;243(130):264–266. doi: 10.1038/newbio243264a0. [DOI] [PubMed] [Google Scholar]
- Darai G., Munk K. Human embryonic lung cells abortively infected with Herpes virus hominis type 2 show some properties of cell transformation. Nat New Biol. 1973 Feb 28;241(113):268–269. doi: 10.1038/newbio241268a0. [DOI] [PubMed] [Google Scholar]
- Diamond L. Two spontaneously transformed cell lines derived from the same hamster embryo culture. Int J Cancer. 1967 Mar 15;2(2):143–152. doi: 10.1002/ijc.2910020209. [DOI] [PubMed] [Google Scholar]
- Duff R., Doller E., Rapp F. Immunologic manipulation of metastases due to herpesvirus transformed cells. Science. 1973 Apr 6;180(4081):79–81. doi: 10.1126/science.180.4081.79. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDDY B. E., BORMAN G. S., GRUBBS G. E., YOUNG R. D. Identification of the oncogenic substance in rhesus monkey kidney cell culture as simian virus 40. Virology. 1962 May;17:65–75. doi: 10.1016/0042-6822(62)90082-x. [DOI] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Hoekstra J., Deinhardt F. Demonstration of Herpesvirus saimiri-associated antigens in peripheral lymphocytes from infected marmosets during in vitro cultivation. J Natl Cancer Inst. 1972 Feb;48(2):523–530. [PubMed] [Google Scholar]
- Fox T. O., Levine A. J. Relationship between virus-induced cellular deoxyribonucleic acid synthesis and transformation by simian virus 40. J Virol. 1971 Apr;7(4):473–477. doi: 10.1128/jvi.7.4.473-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Roizman B., Cassai E., Nahmias A. A DNA fragment of Herpes simplex 2 and its transcription in human cervical cancer tissue. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3784–3789. doi: 10.1073/pnas.69.12.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS L. A filterable agent, recovered from Ak leukemic extracts, causing salivary gland carcinomas in C3H mice. Proc Soc Exp Biol Med. 1953 Jun;83(2):414–421. doi: 10.3181/00379727-83-20376. [DOI] [PubMed] [Google Scholar]
- GROUPE V., MANAKER R. A. Discrete foci of altered chicken embryo cells associated with Rous sarcoma virus in tissue culture. Virology. 1956 Dec;2(6):838–840. doi: 10.1016/0042-6822(56)90064-2. [DOI] [PubMed] [Google Scholar]
- Garfinkle B., McAuslan B. R. Transformation of cultured mammalian cells by viable herpes simplex virus subtypes 1 and 2. Proc Natl Acad Sci U S A. 1974 Jan;71(1):220–224. doi: 10.1073/pnas.71.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geder L., Skinner G. R. Differentiation between type 1 and type 2 strains of herpes simplex virus by an indirect immunofluorescent technique. J Gen Virol. 1971 Aug;12(2):179–182. doi: 10.1099/0022-1317-12-2-179. [DOI] [PubMed] [Google Scholar]
- HABEL K. SPECIFIC COMPLEMENT-FIXING ANTIGENS IN POLYOMA TUMORS AND TRANSFORMED CELLS. Virology. 1965 Jan;25:55–61. doi: 10.1016/0042-6822(65)90251-5. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., TURNER H. C., LANE W. T. SPECIFIC ADENOVIRUS COMPLEMENT-FIXING ANTIGENS IN VIRUS-FREE HAMSTER AND RAT TUMORS. Proc Natl Acad Sci U S A. 1963 Aug;50:379–389. doi: 10.1073/pnas.50.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Hinze H. C., Wegner D. L. Oncogenicity of rabbit herpesvirus. Cancer Res. 1973 Jun;33(6):1434–1435. [PubMed] [Google Scholar]
- Hunt R. D., Meléndez L. V. Clinical, epidemiologic, and pathologic features of cytocidal and oncogenic herpesviruses in South American monkeys. J Natl Cancer Inst. 1972 Jul;49(1):261–271. [PubMed] [Google Scholar]
- Hunt R. D., Meléndez L. V., King N. W., Garcia F. G. Herpesvirus saimiri malignant lymphoma in spider monkeys. A new susceptible host. J Med Primatol. 1972;1(2):114–128. doi: 10.1159/000460372. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Macpherson I. The basis of the tumorigenicity of BHK 21 cells. Int J Cancer. 1968 Sep 15;3(5):654–662. doi: 10.1002/ijc.2910030514. [DOI] [PubMed] [Google Scholar]
- Kutinová L., Vonka V., Broucek J. Increased oncogenicity and synthesis of herpesvirus antigens in hamster cells exposed to herpes simplex type-2 virus. J Natl Cancer Inst. 1973 Mar;50(3):759–766. doi: 10.1093/jnci/50.3.759. [DOI] [PubMed] [Google Scholar]
- Latarjet R., Cramer R., Montagnier L. Inactivation, by UV-, x-, and gamma-radiations, of the infecting and transforming capacities of polyoma virus. Virology. 1967 Sep;33(1):104–111. doi: 10.1016/0042-6822(67)90098-0. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Macnab J. C. Transformation of rat embryo cells by temperature-sensitive mutants of herpes simplex virus. J Gen Virol. 1974 Jul;24(1):143–153. doi: 10.1099/0022-1317-24-1-143. [DOI] [PubMed] [Google Scholar]
- Macpherson I., Russell W. Transformations in hamster cells mediated by mycoplasmas. Nature. 1966 Jun 25;210(5043):1343–1345. doi: 10.1038/2101343a0. [DOI] [PubMed] [Google Scholar]
- Muñoz N. Effect of herpesvirus type 2 and hormonal imbalance on the uterine cervix of the mouse. Cancer Res. 1973 Jun;33(6):1504–1508. [PubMed] [Google Scholar]
- Nadkarni J. S., Nadkarni J. J., Klein G., Henle W., Henle G., Clifford P. EB viral antigens in Burkitt tumor biopsies and early cultures. Int J Cancer. 1970 Jul 15;6(1):10–17. doi: 10.1002/ijc.2910060103. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Josey W. E., Naib Z. M., Luce C. F., Duffey A. Antibodies to Herpesvirus hominis types 1 and 2 in humans. I. Patients with genital herpetic infections. Am J Epidemiol. 1970 Jun;91(6):539–546. doi: 10.1093/oxfordjournals.aje.a121165. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Naib Z. M., Josey W. E., Murphy F. A., Luce C. F. Sarcomas after inoculation of newborn hamsters with Herpes virus hominis type 2 strains. Proc Soc Exp Biol Med. 1970 Sep;134(4):1065–1069. doi: 10.3181/00379727-134-34945. [DOI] [PubMed] [Google Scholar]
- Naib Z. M., Nahmias A. J., Josey W. E. Cytology and histopathology of cervical herpes simplex infection. Cancer. 1966 Jul;19(7):1026–1031. doi: 10.1002/1097-0142(196607)19:7<1026::aid-cncr2820190718>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Plummer G., Goodheart C. R., Miyagi M., Skinner G. R., Thouless M. E., Wildy P. Herpes simplex viruses: discrimination of types and correlation between different characteristics. Virology. 1974 Jul;60(1):206–216. doi: 10.1016/0042-6822(74)90378-x. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Identification of the filtrable leukocyte-transforming factor of QIMR-WIL cells as herpes-like virus. Int J Cancer. 1969 May 15;4(3):255–260. doi: 10.1002/ijc.2910040302. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Tompkins W. A., Figueroa M. E., Melnick J. L. Herpesvirus type 2: association with carcinoma of the cervix. Science. 1968 Sep 20;161(3847):1255–1256. doi: 10.1126/science.161.3847.1255. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Smith J. B. Triploidy in human cell line. Science. 1965 Sep 24;149(3691):1516–1517. doi: 10.1126/science.149.3691.1516. [DOI] [PubMed] [Google Scholar]
- Ross L. J., Watson D. H., Wildy P. Development and localization of virus-specific antigens during the multiplication of herpes simplex virus in BHK 21 cells. J Gen Virol. 1968 Jan;2(1):115–122. doi: 10.1099/0022-1317-2-1-115. [DOI] [PubMed] [Google Scholar]
- Royston I., Aurelian L. Immunofluorescent detection of herpesvirus antigens in exfoliated cells from human cervical carcinoma. Proc Natl Acad Sci U S A. 1970 Sep;67(1):204–212. doi: 10.1073/pnas.67.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVET-MOLDAVSKY G. J. Sarcoma in albino rats treated during the embryonic stage with Rous virus. Nature. 1958 Nov 22;182(4647):1452–1453. doi: 10.1038/1821452b0. [DOI] [PubMed] [Google Scholar]
- Skinner G. R., Thouless M. E., Jordan J. A. Antibodies to type 1 and type 2 herpes virus in women with abnormal cervical cytology. J Obstet Gynaecol Br Commonw. 1971 Nov;78(11):1031–1038. doi: 10.1111/j.1471-0528.1971.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Scher C. D., Todaro G. J. Induction of cell division in medium lacking serum growth factor by SV40. Virology. 1971 May;44(2):359–370. doi: 10.1016/0042-6822(71)90267-4. [DOI] [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Yamanishi K. Transformation of hamster embryo and human embryo cells by temperature sensitive mutants of herpes simplex virus type 2. Virology. 1974 Sep;61(1):306–311. doi: 10.1016/0042-6822(74)90267-0. [DOI] [PubMed] [Google Scholar]
- Tsuda T. Establishment of two cell lines from Syrian hamster embryonic tissue in vitro. Tohoku J Exp Med. 1965 Sep 25;86(4):380–393. doi: 10.1620/tjem.86.380. [DOI] [PubMed] [Google Scholar]
- VANTSIS J. T., WILDY P. Interaction of herpes virus and HeLa cells: comparison of cell killing and infective center formation. Virology. 1962 Jun;17:225–232. doi: 10.1016/0042-6822(62)90112-5. [DOI] [PubMed] [Google Scholar]
- WILDY P. Recombination with herpes simplex virus. J Gen Microbiol. 1955 Oct;13(2):346–360. doi: 10.1099/00221287-13-2-346. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Shedden W. I., Elliot A., Tetsuka T., Wildy P., Bourgaux-Ramoisy D., Gold E. Virus specific antigens in mammalian cells infected with herpes simplex virus. Immunology. 1966 Oct;11(4):399–408. [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Macnab J. C., Subak-Sharpe J. H. The structure and biological properties of herpes simplex virus DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):657–666. doi: 10.1101/sqb.1974.039.01.079. [DOI] [PubMed] [Google Scholar]
- Yamane I., Tsuda T. Malignant transformation and chromosome change of two cell lines from Syrian hamster embryonic tissue in vitro. Tohoku J Exp Med. 1966 Feb 25;88(2):171–180. doi: 10.1620/tjem.88.171. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H., Schulte-Holthausen H. Presence of EB virus nucleic acid homology in a "virus-free" line of Burkitt tumour cells. Nature. 1970 Jul 18;227(5255):245–248. doi: 10.1038/227245a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970 Dec 12;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]