Abstract
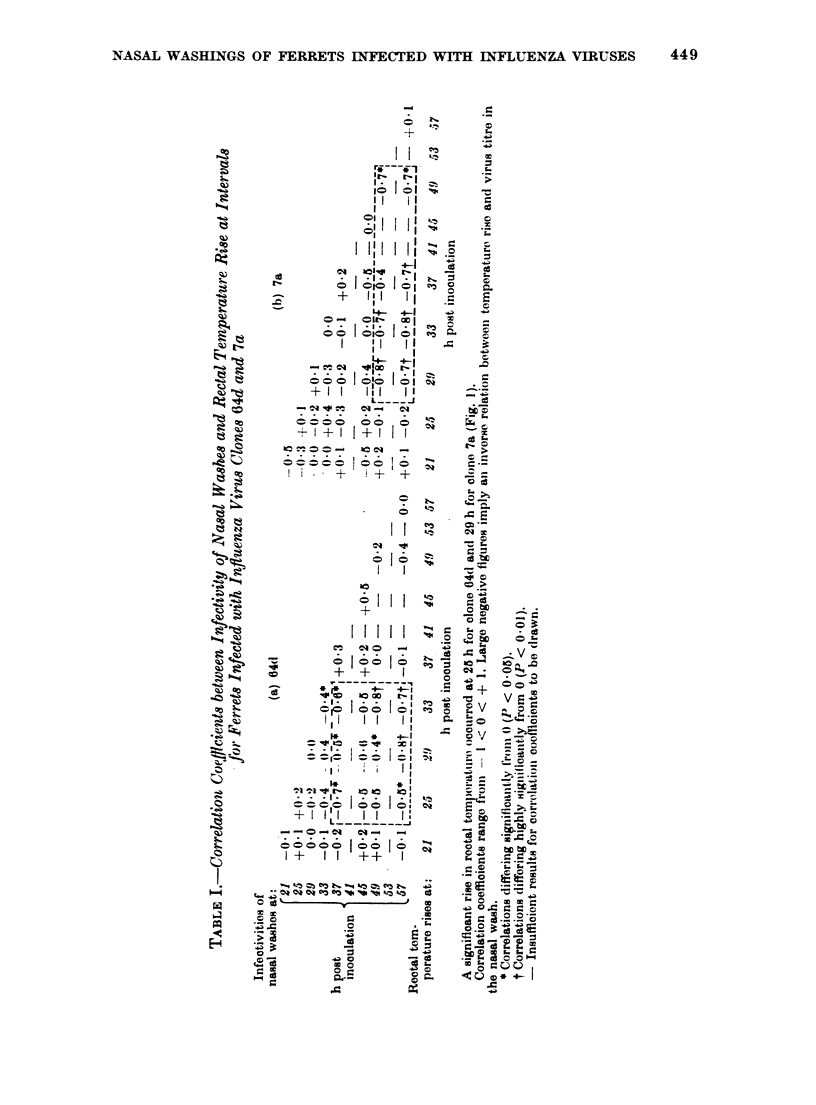
Virus titres in the nasal washes of ferrets infected with recombinant influenza virus A/PR/8/34-A/England/939/69 (H3N2) clones 7a (virulent) and 64d (attenuated) showed four phases; a phase of logarithmic increase, a plateau, a period of rapid decline and a small rise on the 4th day. The virulent clone remained in the phase of logarithmic increase longer, reached plateau levels 10-fold higher and took longer to reduce to low levels than the attenuated clone. Pyrexia (more severe and prolonged for clone 7a) and inflammatory response (similar for both clones, consisting of 90% polymorphonuclear (PMN) and 10% mononuclear (MN) phagocytes) occurred at about the same time (25-29 h after inoculation) for both clones. However, the onset of the period of rapid decline of clone 64d in the nasal washes occurred 4-8 h before that for 7a in 11 of 16 ferrets. Furthermore, significant correlations were found between increases in temperature and numbers of inflammatory cells and subsequent reductions in virus titres in nasal washes; and the delay between the responses and subsequent correlated reductions of virus was less for clone 64d than for clone 7a. Thus, a link between temperature rise and inflammatory response and subsequent reduction of virus titre was detected and, whatever the mechanisms of this link, it acted differentially on the clones of differing virulence. However, the period of logarithmic increase of clone 64d ended before pyrexia and inflammation were detected and, in the plateau stage at the onset of the host responses, there were no significant correlations between them and virus levels measured at the same time or shortly afterwards. Pyrexia and inflammation also seemed to be connected with decline of virus recoveries for infections with virus strains A/PR/8/34 (HoN1) and A/New Jersey/18/76 (Hsw15N1).
The significance of the relations between pyrexia and cell response and decline of virus titres in nasal washings is discussed.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKMON J. R., GINSBERG H. S. Reactions of influenza viruses with guinea pig polymorphonuclear leucocytes. I. Virus-cell interactions. Virology. 1956 Oct;2(5):618–636. doi: 10.1016/0042-6822(56)90043-5. [DOI] [PubMed] [Google Scholar]
- BOAND A. V., Jr, KEMPF J. E., HANSON R. J. Phagocytosis of influenza virus. I. In vitro observations. J Immunol. 1957 Nov;79(5):416–421. [PubMed] [Google Scholar]
- Beare A. S., Hall T. S. Recombinant influenza-A viruses as live vaccines for man. Report to the Medical Research Council's Committee on Influenza and other Respiratory Virus Vaccines. Lancet. 1971 Dec 11;2(7737):1271–1273. doi: 10.1016/s0140-6736(71)90597-6. [DOI] [PubMed] [Google Scholar]
- DAVENPORT F. M. Pathogenesis of influenza. Bacteriol Rev. 1961 Sep;25:294–300. doi: 10.1128/br.25.3.294-300.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haff R. F., Schriver P. W., Stewart R. C. Pathogenesis of influenza in ferrets: nasal manifestations of disease. Br J Exp Pathol. 1966 Oct;47(5):435–444. [PMC free article] [PubMed] [Google Scholar]
- Kolot F. B., Baron S., Yeager H., Jr, Schwartz S. L. Comparative production of interferon by explanted lymphoreticular tissue and alveolar macrophages from rabbits and humans. Infect Immun. 1976 Jan;13(1):63–68. doi: 10.1128/iai.13.1.63-68.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H. E., Blades R. Impairment of human polymorphonuclear leucocyte function by influenza virus. Lancet. 1976 Feb 7;1(7954):283–283. doi: 10.1016/s0140-6736(76)91407-0. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Oxford J. S., Shore S. L., McLaren C., Stuart-Harris C. Immunity to influenza in ferrets. I. Response to live and killed virus. Br J Exp Pathol. 1972 Apr;53(2):153–167. [PMC free article] [PubMed] [Google Scholar]
- Sawyer W. D. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J Infect Dis. 1969 Jun;119(6):541–556. doi: 10.1093/infdis/119.6.541. [DOI] [PubMed] [Google Scholar]


