Abstract
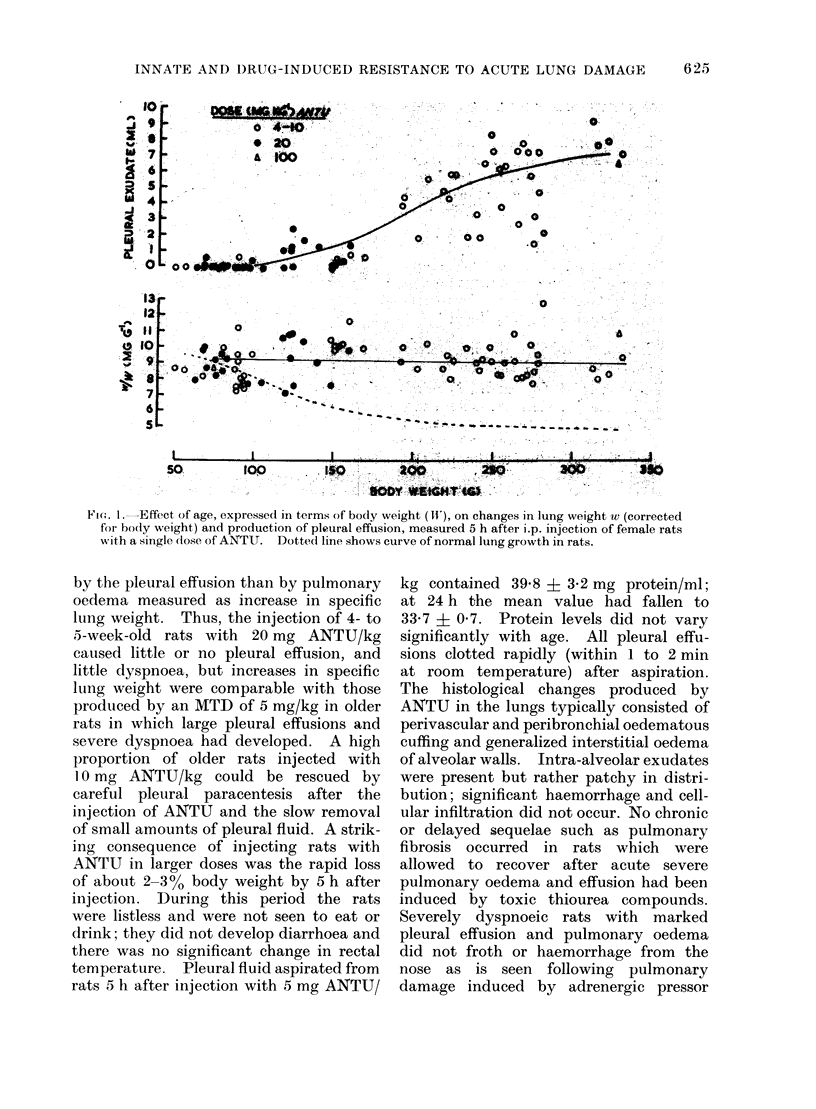
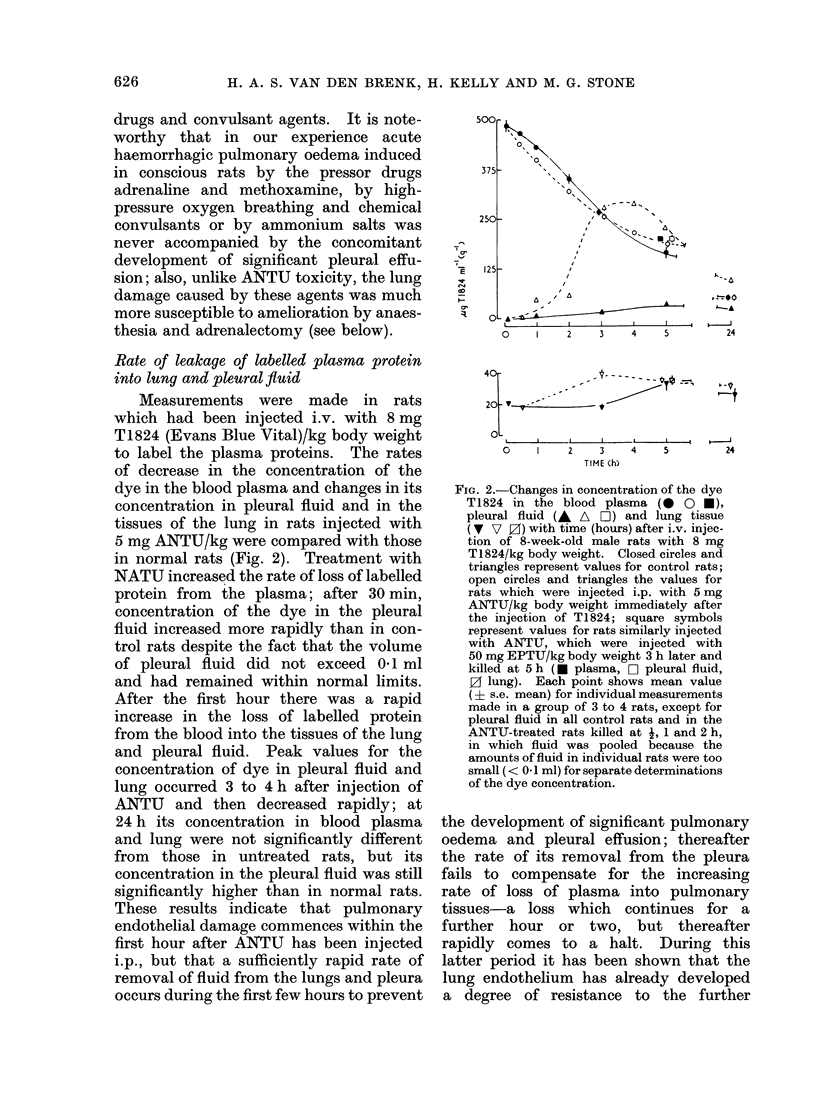
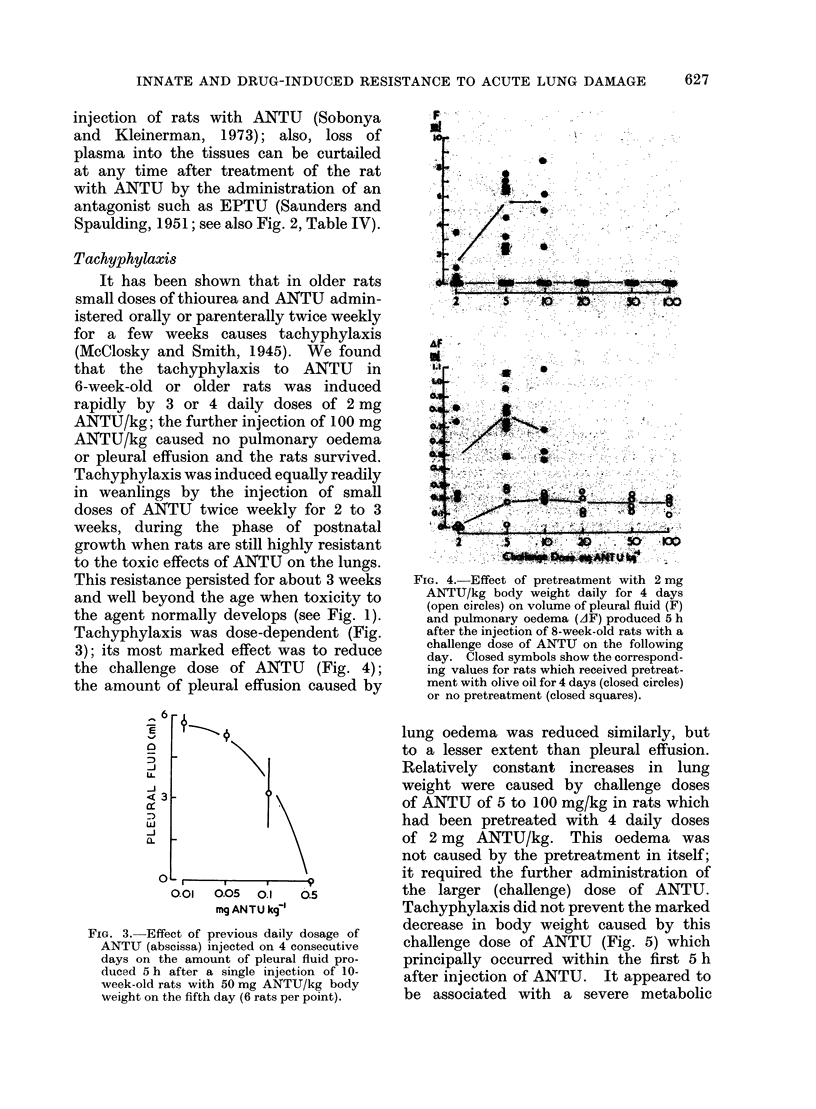
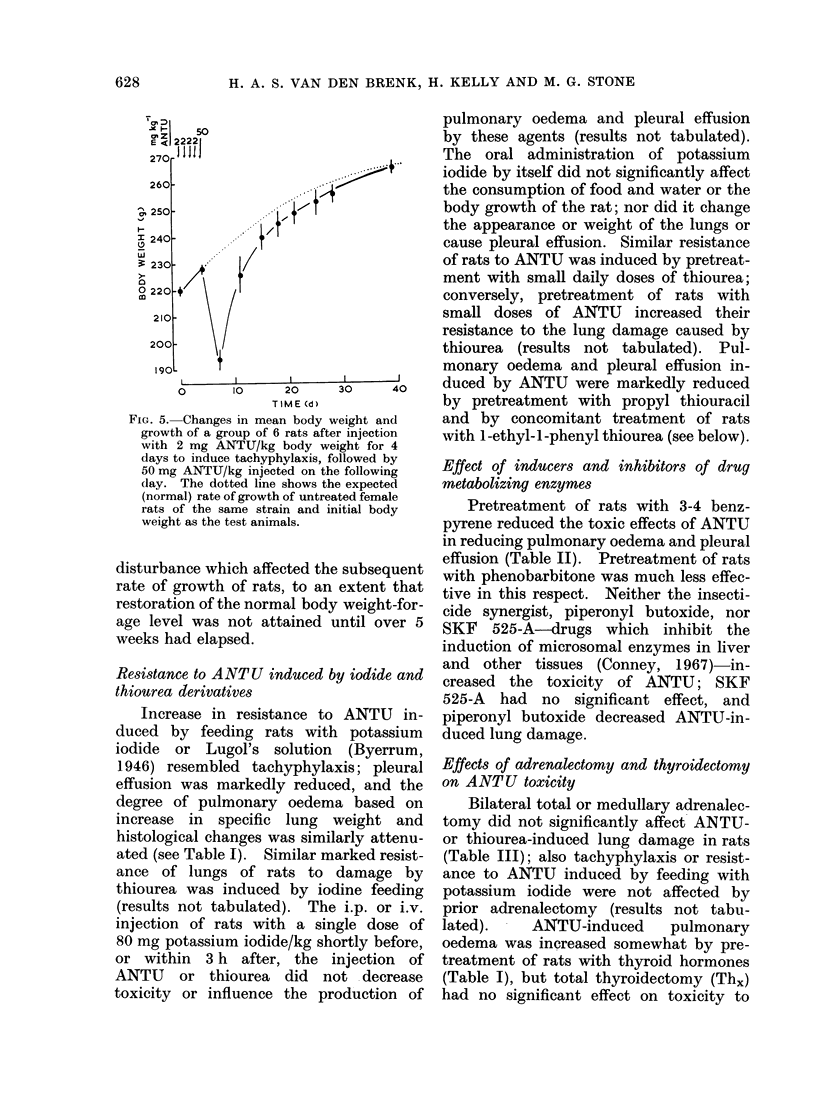
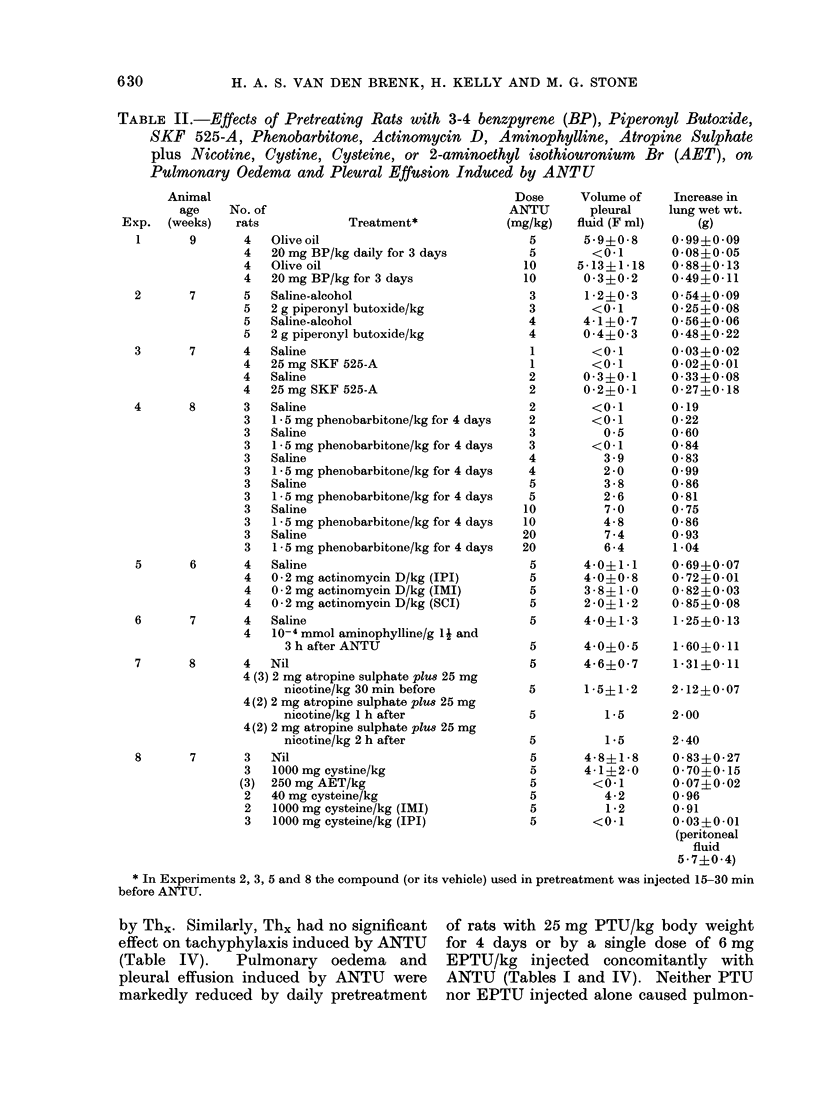
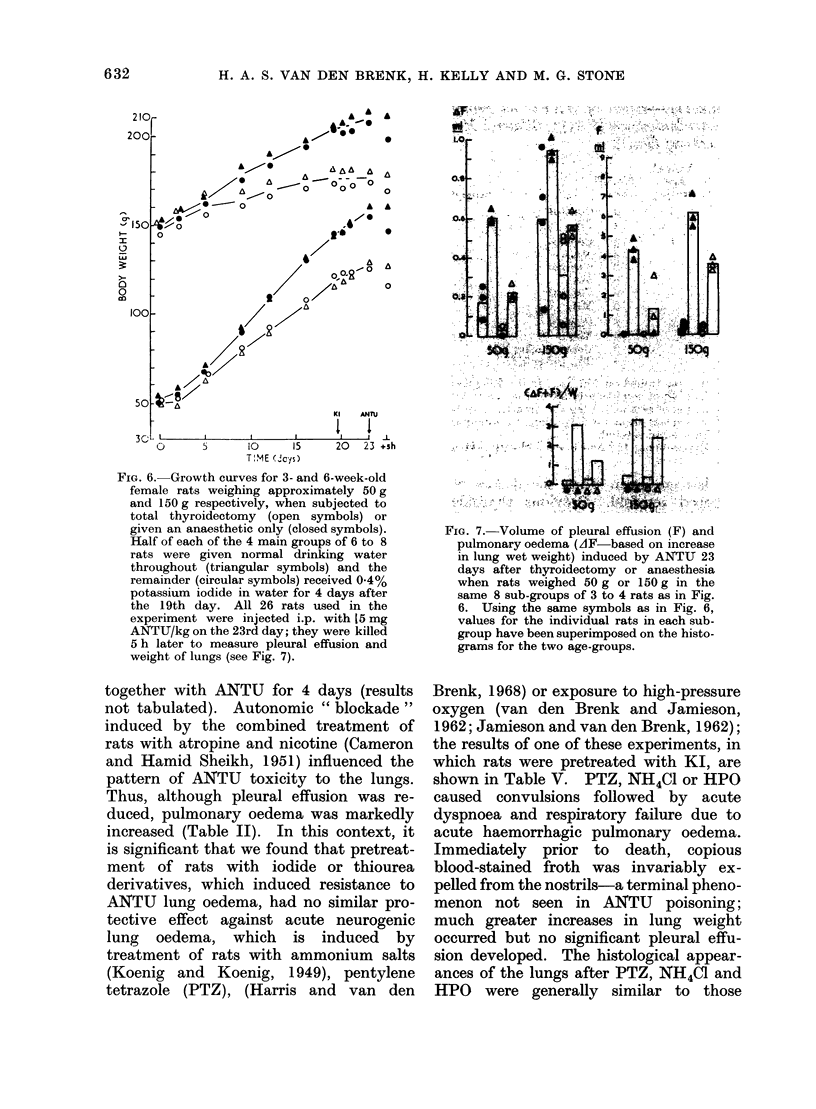
During the 3rd and 4th weeks of life rats were highly resistant to the toxic effects of alpha-naphthyl thiourea (ANTU) and of thiourea and its derivatives but toxicity developed rapidly during the following 2 weeks. Marked resistance to lung damage by toxic thioureas could be induced in older, mature rats by pretreatment with the toxic agent itself (tachyphylaxis), with other toxic and non-toxic antithyroid drugs or with iodine or iodide--even if the rats were pretreated at an early age before susceptibility to the agent developed. ANTU-tachyphylaxis was dose-dependent. Total thyroidectomy did not affect either lung damage induced by ANTU or the resistance due to tachyphylaxis or to pretreatment with iodide or the antithyroid drugs thiourea, 1-ethyl-1-phenyl thiourea or propyl thiouracil. Neither total nor medullary adrenalectomy affected ANTU toxicity. Marked resistance to ANTU-induced lung damage was induced in rats by pretreatment with either an activator (3-4 benzypyrene) or an inhibitor (SKF 525-A) of drug-metabolizine mixed-function microsomal enzyme systems; the inhibitor, sodium phenobarbitone, had no significant effect on toxicity. The sulphydryl compound, AET, induced marked resistance to ANTU; cysteine was less effective. Neither autonomic blockade with nicotine and atropine nor actinomycin D had significant effects on toxicity to ANTU. The acute pulmonary oedema induced in rats by high pressure oxygen, chemical convulsants, pressor agents and ammonium sulphate differed in many respects from that induced by toxic thioureas; it was typically haemorrhagic in nature, did not result in significant pleural effusion, did not exhibit tachyphylaxis, and was not influenced by pretreatment with iodide or derivatives of thiourea.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAN J. W., JOHNSON P. C. Epinephrine and neurogenic factors in the pulmonary edema and CNS reactions induced by O2 at high pressure. Am J Physiol. 1955 Feb;180(2):438–444. doi: 10.1152/ajplegacy.1955.180.2.438. [DOI] [PubMed] [Google Scholar]
- BYERRUM R. U., COCHRAN K. W., DUBOIS K. P. Influence of alpha-naphthylthiourea (ANTU) on iodine metabolism. Proc Soc Exp Biol Med. 1950 Apr;73(4):661–664. doi: 10.3181/00379727-73-17779. [DOI] [PubMed] [Google Scholar]
- Bend J. R., Hook G. E., Easterling R. E., Gram T. E., Fouts J. R. A comparative study of the hepatic and pulmonary microsomal mixed-function oxidase systems in the rabbit. J Pharmacol Exp Ther. 1972 Oct;183(1):206–217. [PubMed] [Google Scholar]
- Butler W. H., Mattocks A. R., Barnes J. M. Lesions in the liver and lungs of rats given pyrrole derivatives of pyrrolizidine alkaloids. J Pathol. 1970 Mar;100(3):169–175. doi: 10.1002/path.1711000305. [DOI] [PubMed] [Google Scholar]
- Böhm G. M. Vascular permeability changes during experimentally produced pulmonary oedema in rats. J Pathol Bacteriol. 1966 Jul;92(1):151–161. doi: 10.1002/path.1700920116. [DOI] [PubMed] [Google Scholar]
- CAMERON G. R., SHEIKH A. H. The experimental production of pulmonary oedema with ammonium salts, together with a classification of lung oedema. J Pathol Bacteriol. 1951 Oct;63(4):609–617. doi: 10.1002/path.1700630407. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Cottrell T. S., Levine O. R., Senior R. M., Wiener J., Spiro D., Fishman A. P. Electron microscopic alterations at the alveolar level in pulmonary edema. Circ Res. 1967 Dec;21(6):783–797. doi: 10.1161/01.res.21.6.783. [DOI] [PubMed] [Google Scholar]
- Cunningham A. L., Hurley J. V. Alpha-naphthyl-thiourea-induced pulmonary oedema in the rat: a topographical and electron-microscope study. J Pathol. 1972 Jan;106(1):25–35. doi: 10.1002/path.1711060103. [DOI] [PubMed] [Google Scholar]
- Giri S. N., Hollinger M. A., Cross C. E., Dungworth D. L. Effects of thiourea on pulmonary edema, pleural and peritoneal effusions and toxicity in rats pretreated with actinomycin D. Toxicology. 1974 Sep;2(3):211–222. doi: 10.1016/0300-483x(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Granner D. K. Restoration of sensitivity of cultured hepatoma cells to cyclic nucleotides shows permissive effect of dexamethasone. Nature. 1976 Feb 19;259(5544):572–573. doi: 10.1038/259572a0. [DOI] [PubMed] [Google Scholar]
- Harris J. W., Van den Brenk H. A. Comparative effects of hyperbaric oxygen and pentylenetetrazol on lung weight and non-protein sulfhydryl content of experimental animals. Biochem Pharmacol. 1968 Jul;17(7):1181–1188. doi: 10.1016/0006-2952(68)90054-3. [DOI] [PubMed] [Google Scholar]
- Hayes J. A., Shiga A. Ultrastructural changes in pulmonary oedema produced experimentally with ammonium sulphate. J Pathol. 1970 Apr;100(4):281–286. doi: 10.1002/path.1711000407. [DOI] [PubMed] [Google Scholar]
- JAMIESON D., VAN DEN BRENK H. A. Pulmonary damage due to high pressure oxygen breathing in rats. Aust J Exp Biol Med Sci. 1962 Aug;40:309–314. doi: 10.1038/icb.1962.34. [DOI] [PubMed] [Google Scholar]
- JUDAH J. D., WILLOUGHBY D. A. A quantitative method for the study of capillary permeability: extraction and determination of trypan blue in tissues. J Pathol Bacteriol. 1962 Apr;83:567–572. doi: 10.1002/path.1700830236. [DOI] [PubMed] [Google Scholar]
- Jenner S., Netter K. J. On the inhibition of microsomal drug metabolism by SKF 525-A. Biochem Pharmacol. 1972 Jul 15;21(14):1921–1927. doi: 10.1016/0006-2952(72)90004-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacKAY E. M., PECKA E. F., Jr Experimental pulmonary edema. III. Hypoglycemia, a cause of pulmonary edema. Proc Soc Exp Biol Med. 1950 Apr;73(4):568–569. doi: 10.3181/00379727-73-17747. [DOI] [PubMed] [Google Scholar]
- RICHTER C. P. The physiology and cytology of pulmonary edema and pleural effusion produced in rats by alpha-naphthyl thiourea (ANTU). J Thorac Surg. 1952 Jan;23(1):66–91. [PubMed] [Google Scholar]
- SAUNDERS J. P., SPAULDING R. C. Effects of some substituted thioureas on alpha-naphthylthiourea (ANTU) toxicity on rats. Proc Soc Exp Biol Med. 1951 Jan;76(1):84–85. doi: 10.3181/00379727-76-18394. [DOI] [PubMed] [Google Scholar]
- Sobonya R. E., Kleinerman J. Recurrent pulmonary edema induced by alpha 1-naphthyl thiourea. Am Rev Respir Dis. 1973 Oct;108(4):926–932. doi: 10.1164/arrd.1973.108.4.926. [DOI] [PubMed] [Google Scholar]
- Van Den Brenk H. A., Stone M., Kelly H., Orton C., Sharpington C. Promotion of growth of tumour cells in acutely inflamed tissues. Br J Cancer. 1974 Sep;30(3):246–260. doi: 10.1038/bjc.1974.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebel F. J., Bader J. P., Gelboin H. V. Enzyme induction in mammalian cells defective in 28S ribosomal RNA formation. Nature. 1976 Jan 29;259(5541):331–333. doi: 10.1038/259331a0. [DOI] [PubMed] [Google Scholar]
- van den Brenk H. A., Sparrow N., Moore V. Effect of x-radiation on salivary gland growth in the rat. I. Effect of single doses on post-natal differentiation and growth of acinar and duct components. Int J Radiat Biol Relat Stud Phys Chem Med. 1969;16(3):241–266. doi: 10.1080/09553006914551261. [DOI] [PubMed] [Google Scholar]


