Abstract
After intranasal inoculation of ferrets with influenza virus the upper respiratory tract infection diminishes during the second day and the onset of this reduction occurs earlier for an attenuated clone (64d) than for a virulent clone (7a) of the recombinant virus A/PR/8/34-A/England/939/69 (H3N2). The relevance of pyrexia and the nasal inflammatory response to this reduction in infection has been investigated.
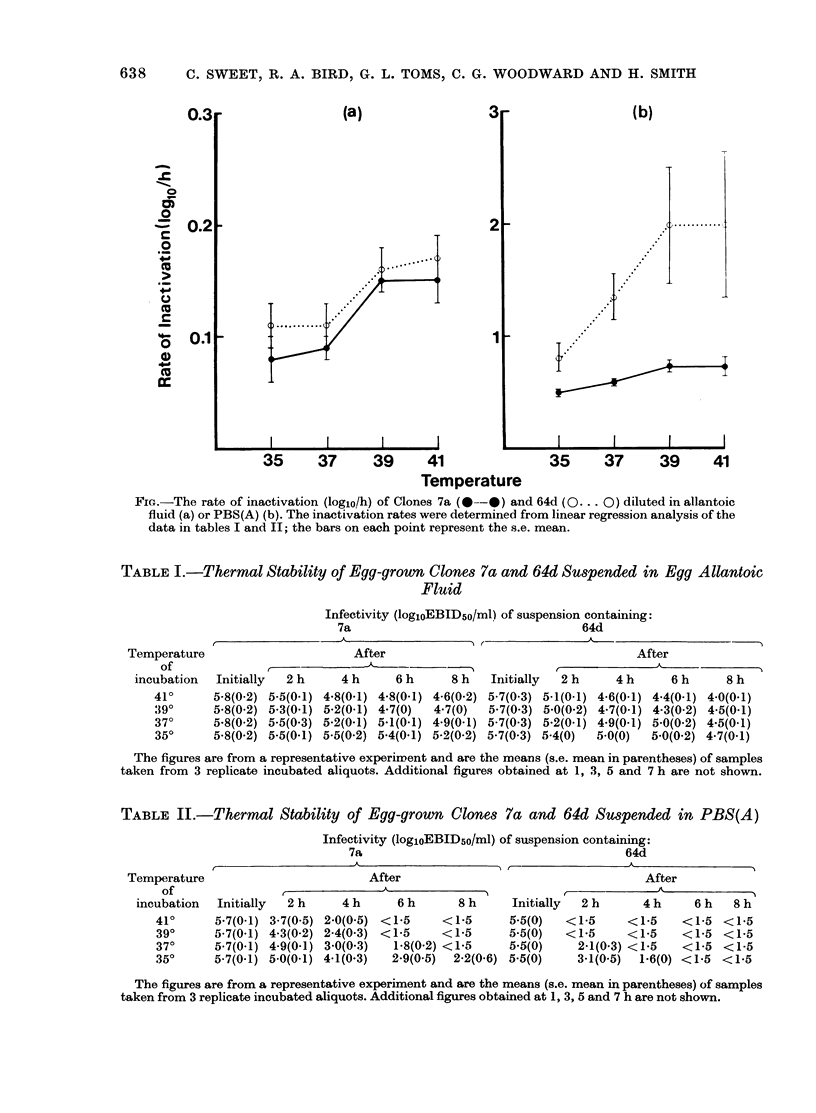
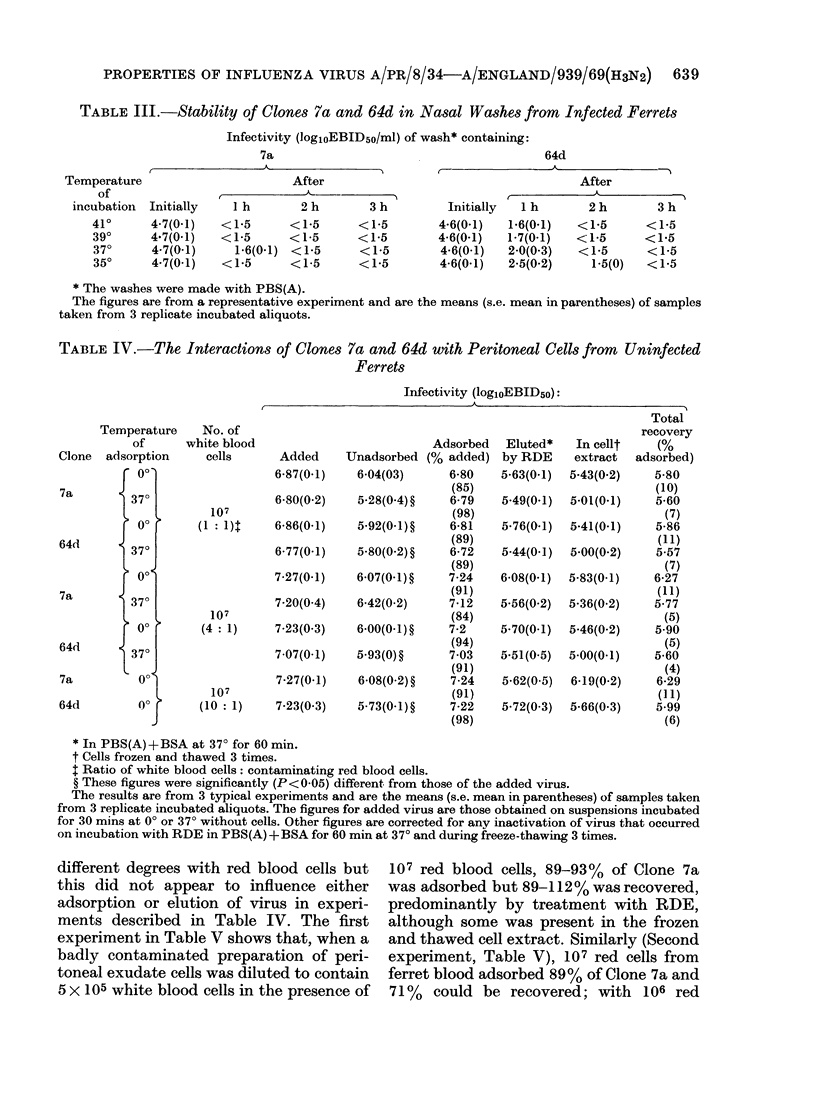
Egg-grown Clone 64d was more thermobile than Clone 7a at normal and pyrexial temperatures when suspended in egg allantoic fluid or phosphate-buffered saline. However, in infected nasal washes, both clones were rapidly inactivated when the washes were incubated at these temperatures.
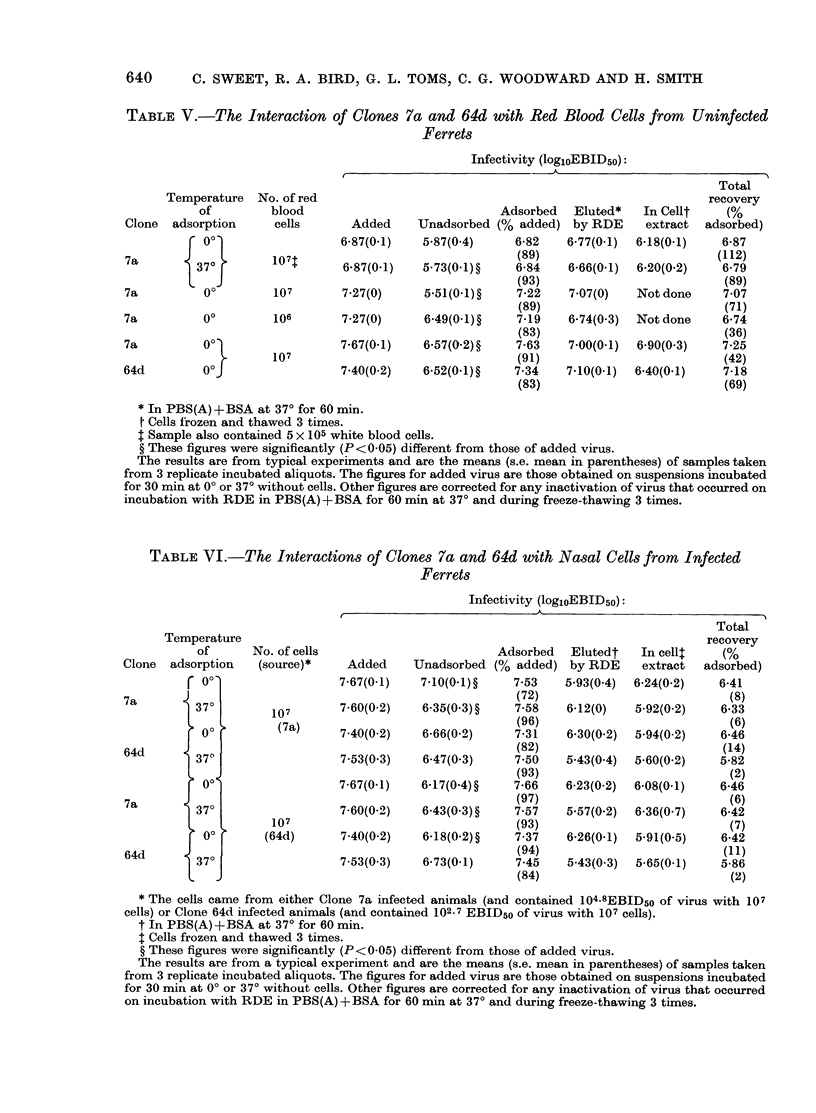
In vitro tests showed that both clones adsorbed to the phagocytes of peritoneal exudates from uninfected ferrets and nasal inflammatory exudates of ferrets infected with both clones. About 90% of the virus was adsorbed after 30 min at 0° or 370° and only 2-14% of this was recovered after treatment with receptor-destroying enzyme followed by freeze-thawing the cells. In contrast, high recoveries (36-112% of that adsorbed) were obtained from red blood cells that were treated similarly. Significant differences were not detected between the clones in either adsorption by or recovery from phagocytes of the different types of exudates.
Thus pyrexia and the nasal inflammatory cells probably play a major role in the reduction of nasal tract infection but, while pyrexia may have had some influence, no evidence was obtained to indicate that the cells contributed to the earlier reduction of Clone 64d.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrescu M., Handrache L., Moroşanu V., Vancea G., Ionescu L., Colev A. Influenza viruses and lympho-macrophagic cells. I. In vitro interaction between influenza A2-59 virus (H2N2) and peritoneal macrophagic cells from normal or immunized mice and rats. Rev Roum Virol. 1974;25(1):3–14. [PubMed] [Google Scholar]
- BLACKMON J. R., GINSBERG H. S. Reactions of influenza viruses with guinea pig polymorphonuclear leucocytes. I. Virus-cell interactions. Virology. 1956 Oct;2(5):618–636. doi: 10.1016/0042-6822(56)90043-5. [DOI] [PubMed] [Google Scholar]
- BOAND A. V., Jr, KEMPF J. E., HANSON R. J. Phagocytosis of influenza virus. I. In vitro observations. J Immunol. 1957 Nov;79(5):416–421. [PubMed] [Google Scholar]
- DAVENPORT F. M. Pathogenesis of influenza. Bacteriol Rev. 1961 Sep;25:294–300. doi: 10.1128/br.25.3.294-300.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. J., KEMPF J. E., BOAND A. V., Jr Phagocytosis of influenza virus. II. Its occurrence in normal and immune mice. J Immunol. 1957 Nov;79(5):422–427. [PubMed] [Google Scholar]
- Hackemann M. M., Denman A. M., Tyrrell D. A. Inactivation of influenza virus by human lymphocytes. Clin Exp Immunol. 1974 Apr;16(4):583–591. [PMC free article] [PubMed] [Google Scholar]
- Kolot F. B., Baron S., Yeager H., Jr, Schwartz S. L. Comparative production of interferon by explanted lymphoreticular tissue and alveolar macrophages from rabbits and humans. Infect Immun. 1976 Jan;13(1):63–68. doi: 10.1128/iai.13.1.63-68.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H. E., Blades R. Impairment of human polymorphonuclear leucocyte function by influenza virus. Lancet. 1976 Feb 7;1(7954):283–283. doi: 10.1016/s0140-6736(76)91407-0. [DOI] [PubMed] [Google Scholar]
- Sawyer W. D. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J Infect Dis. 1969 Jun;119(6):541–556. doi: 10.1093/infdis/119.6.541. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Ernst C., Weinstein L. Letter: Inhibition of human neutrophil chemotaxis by influenza virus. Lancet. 1976 Mar 20;1(7960):650–651. doi: 10.1016/s0140-6736(76)90471-2. [DOI] [PubMed] [Google Scholar]
- Smith H. Mechanisms of virus pathogenicity. Bacteriol Rev. 1972 Sep;36(3):291–310. doi: 10.1128/br.36.3.291-310.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms G. L., Bird R. A., Kingsman S. M., Sweet C., Smith H. The behaviour in ferrets of two closely related clones of influenza virus of differing virulence for man. Br J Exp Pathol. 1976 Feb;57(1):37–48. [PMC free article] [PubMed] [Google Scholar]
- Toms G. L., Davies J. A., Woodward C. G., Sweet C., Smith H. The relation of pyrexia and nasal inflammatory response to virus levels in nasal washings of ferrets infected with influenza viruses of differing virulence. Br J Exp Pathol. 1977 Aug;58(4):444–458. [PMC free article] [PubMed] [Google Scholar]


