Abstract
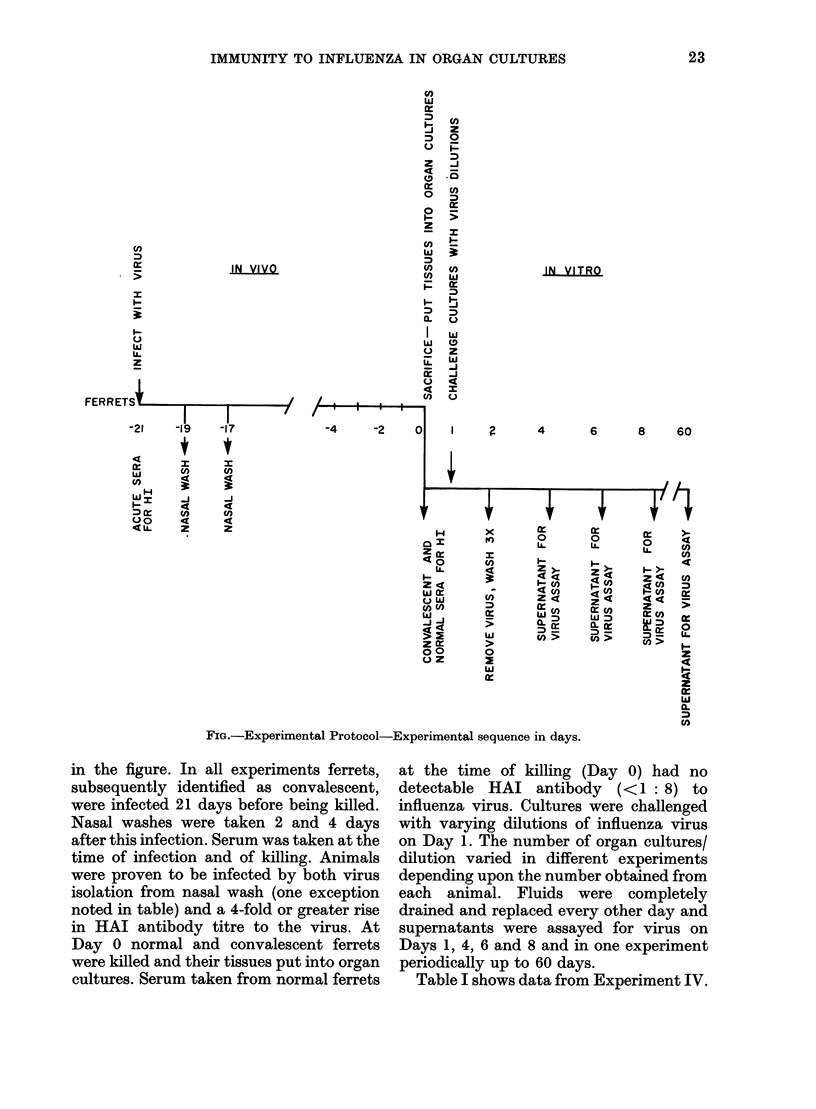
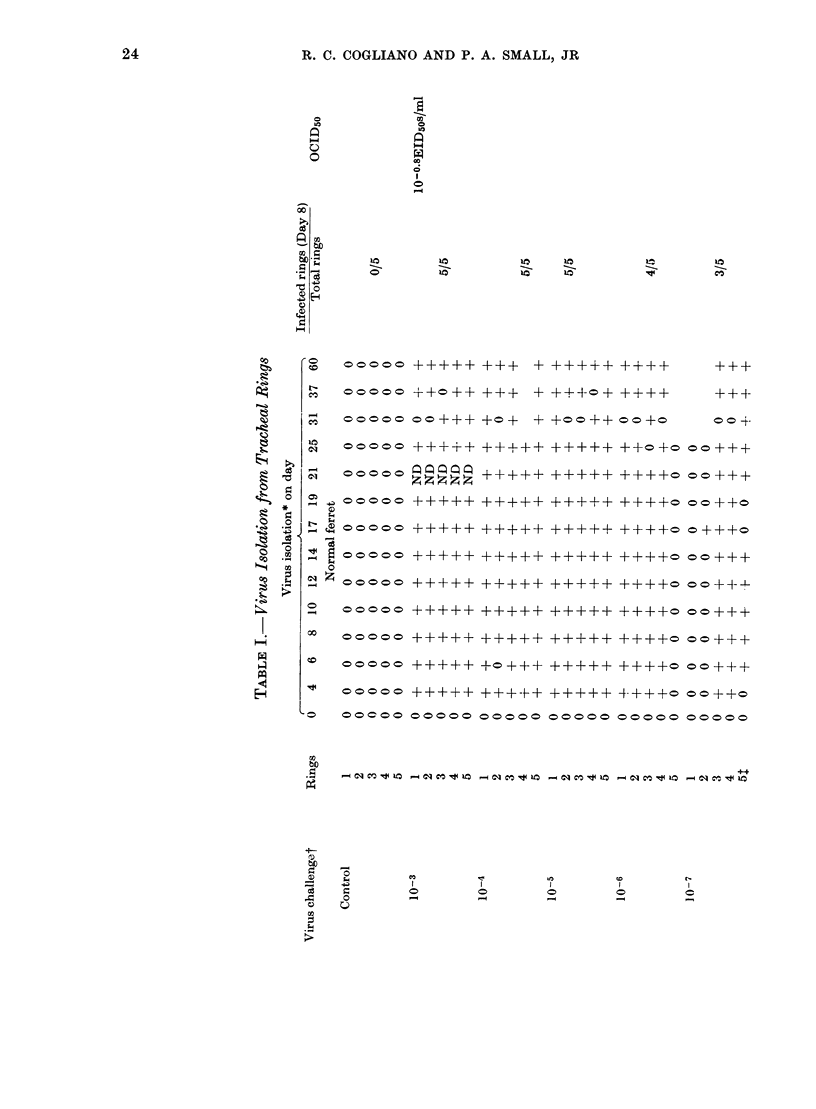
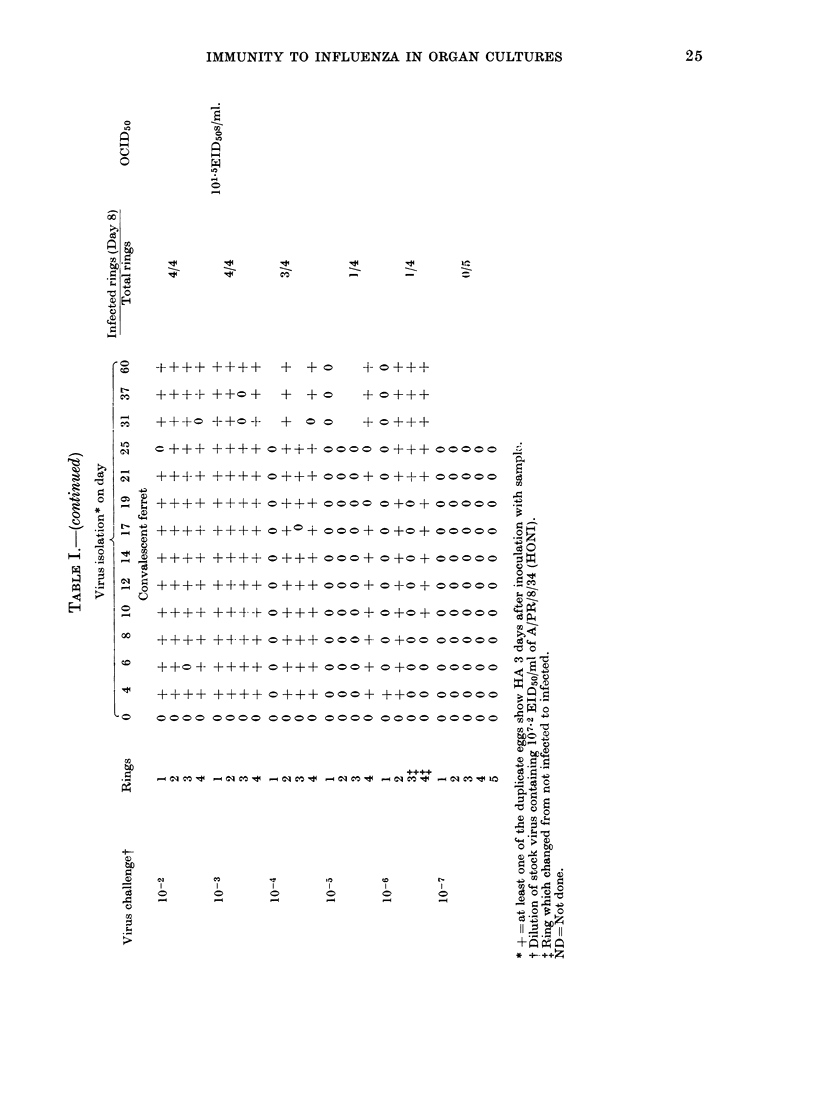
Ferret tracheal organ cultures prepared from animals previously infected intranasally with influenza A virus required approximately 130 times more homologous virus (A/PR/8/34(HON1) or A/Port Chalmers/1/73 (H3N2)) to become infected in vitro than similar cultures from normal ferrets. Also, these cultures from convalescent ferrets required 9 times more heterologous virus (A/PR/8/34(HON1) or Sendai) to become infected in vitro than similar cultures from normal animals challenged in vitro with the hererologous virus. We conclude that these tracheal rings are specifically immune. Once tracheal rings are infected, they continue to shed viruses for at least 60 days, the longest period any cultures were kept. Virus sheeding in the intact ferret lasts normally 5-7 days. Thus recovery in the intact ferret seems to be dependent upon factors which are not present, or at least not functional, in the tracheal explant. This is consistent with the hypothesis that recovery is dependent upon systemic rather than local phenomena. Bladder tissue from normal and previously infected ferrets was also cultured and challenged with homologous and heterologous virus. The bladder from previously infected ferrets exhibited specific immunity, although the immunity was more varible.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan W. H., Madeley C. R., Kendal A. P. Studies with avian influenza A viruses: cross protection experiments in chickens. J Gen Virol. 1971 Aug;12(2):79–84. doi: 10.1099/0022-1317-12-2-79. [DOI] [PubMed] [Google Scholar]
- BANG F. B., NIVEN J. S. A study of infection in organised tissue cultures. Br J Exp Pathol. 1958 Jun;39(3):317–322. [PMC free article] [PubMed] [Google Scholar]
- Barber W. H., Small P. A., Jr Dissemination of influenza virus between anatomically isolated sites in ferrets. Infect Immun. 1974 Mar;9(3):530–533. doi: 10.1128/iai.9.3.530-533.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarab O., Smith H. Growth patterns of influenza virus in cultures of ferret organs. Br J Exp Pathol. 1970 Feb;51(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOORN B., TYRRELL D. A. ON THE GROWTH OF CERTAIN "NEWER" RESPIRATORY VIRUSES IN ORGAN CULTURES. Br J Exp Pathol. 1965 Apr;46:109–118. [PMC free article] [PubMed] [Google Scholar]
- Klein J. D., Collier A. M. Pathogenesis of human parainfluenza type 3 virus infection in hamster tracheal organ culture. Infect Immun. 1974 Oct;10(4):883–888. doi: 10.1128/iai.10.4.883-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren C., Potter C. W., Jennings R. Immunity to influenza in ferrets. 13. Protection against influenza infection by serum antibody to homologous haemagglutinin or neuraminidase antigens. Med Microbiol Immunol. 1974;160(1):33–45. doi: 10.1007/BF02124341. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Kasel J. A., Saglam M., Knight V., Loda F. A. Immunity to influenza to antibody levels. N Engl J Med. 1966 Mar 10;274(10):527–535. doi: 10.1056/NEJM196603102741001. [DOI] [PubMed] [Google Scholar]
- NAFICY K. HUMAN INFLUENZA INFECTION WITH PROVED VIREMIA. REPORT OF A CASE. N Engl J Med. 1963 Oct 31;269:964–966. doi: 10.1056/NEJM196310312691807. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Shore S. L., McLaren C., Stuart-Harris C. Immunity to influenza in ferrets. II. Influence of adjuvants on immunization. Br J Exp Pathol. 1972 Apr;53(2):168–179. [PMC free article] [PubMed] [Google Scholar]
- Rott R., Becht H., Orlich M. The significance of influenza virus neuraminidase in immunity. J Gen Virol. 1974 Jan;22(1):35–41. doi: 10.1099/0022-1317-22-1-35. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Schmidt R. C., Maassab H. F., Davenport F. M. Infection by influenza A viruses of tracheal organ cultures derived from homologous and heterologous hosts. J Infect Dis. 1974 Jan;129(1):28–36. doi: 10.1093/infdis/129.1.28. [DOI] [PubMed] [Google Scholar]
- Schmidt R. C., Maassab H. F. Local immunity to influenza virus in chicken tracheal organ cultures. J Infect Dis. 1974 Jun;129(6):637–643. doi: 10.1093/infdis/129.6.637. [DOI] [PubMed] [Google Scholar]
- Small P. A., Jr, Waldman R. H., Bruno J. C., Gifford G. E. Influenza infection in ferrets: role of serum antibody in protection and recovery. Infect Immun. 1976 Feb;13(2):417–424. doi: 10.1128/iai.13.2.417-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F., Oya J., Ishida N. Effect of antilymphocyte serum on influenza virus infection in mice. Proc Soc Exp Biol Med. 1974 May;146(1):78–84. doi: 10.3181/00379727-146-38047. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L. Host defenses against influenza virus: the role of anti-hemagglutinin antibody. J Immunol. 1975 Aug;115(2):434–439. [PubMed] [Google Scholar]
- Waldman R. H., Coggins W. J. Influenza immunization: field trial on a university campus. J Infect Dis. 1972 Sep;126(3):242–248. doi: 10.1093/infdis/126.3.242. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Courtneidge S. A., Skehel J. J., Crumpton M. J., Askonas B. A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature. 1977 May 26;267(5609):354–356. doi: 10.1038/267354a0. [DOI] [PubMed] [Google Scholar]


