Abstract
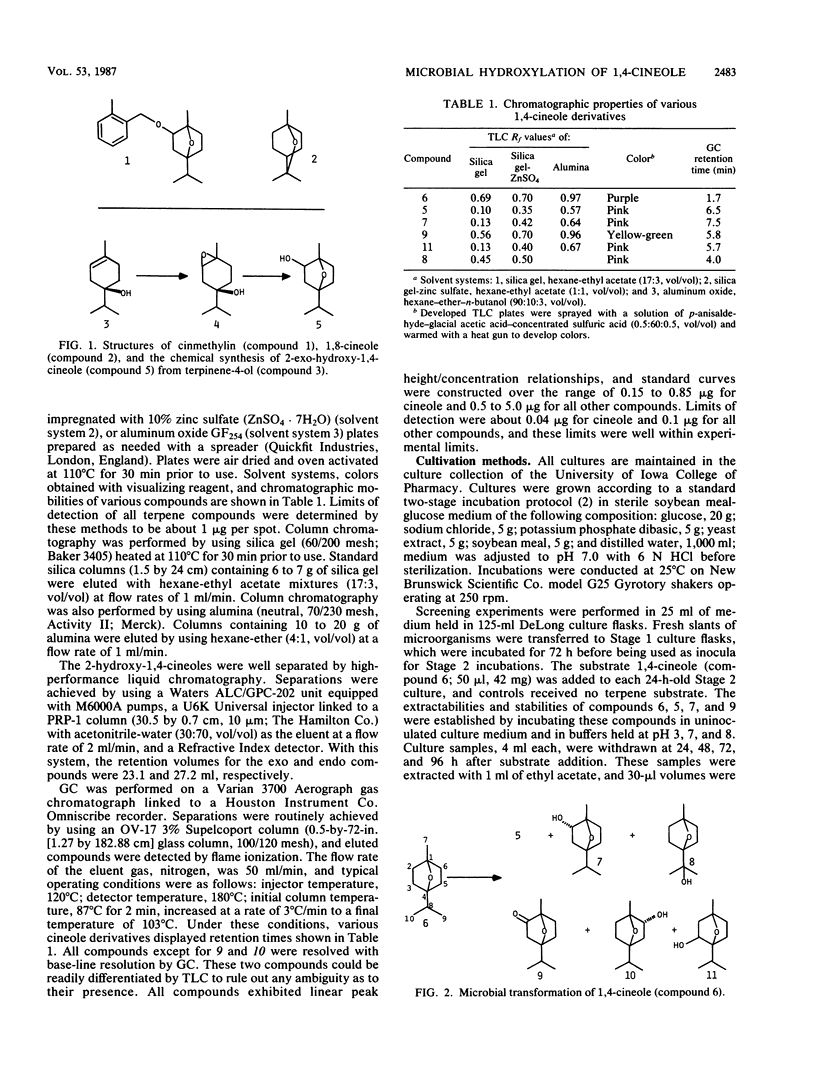
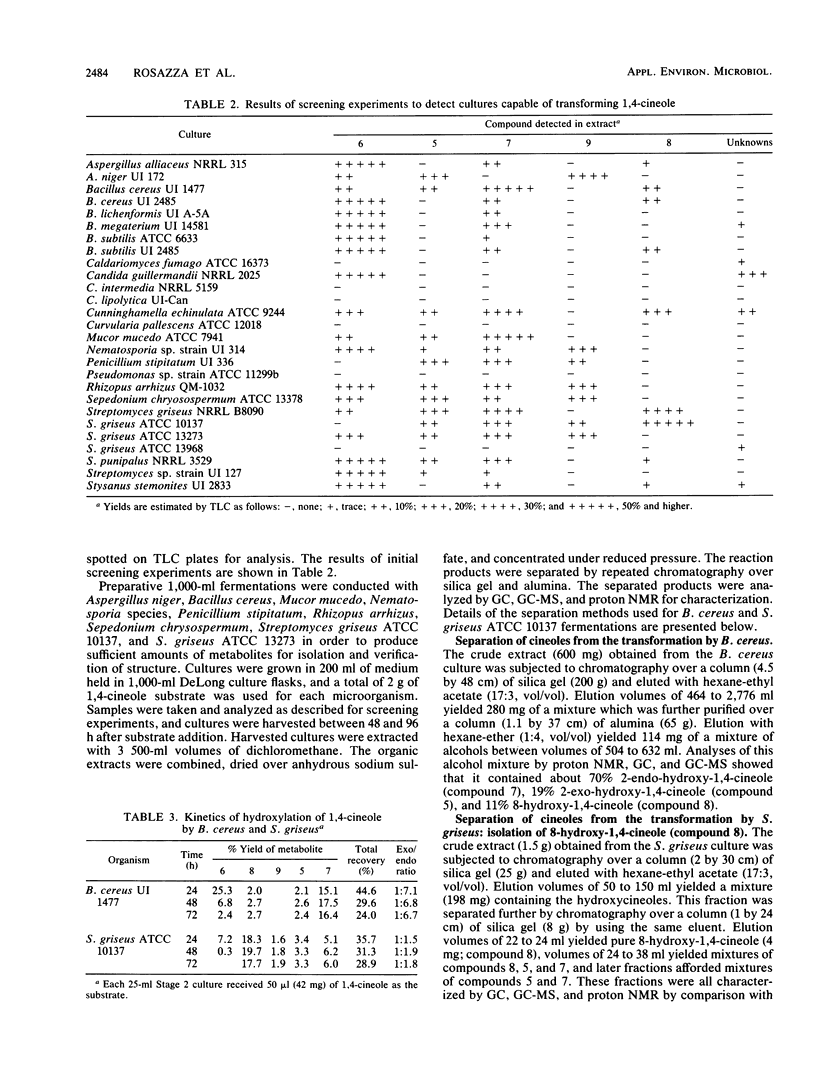
Microorganisms were examined for their potential to hydroxylate the oxygenated monoterpene 1,4-cineole. Using gas chromatography and thin-layer chromatography, screening experiments revealed that hydroxylation at position 2 was the most commonly observed microbial transformation reaction. In most microorganisms, the predominant alcohol metabolite was the 2-endo-alcohol isomer. Preparative-scale incubations were conducted in order to isolate and characterize microbial transformation products by comparison of proton nuclear magnetic resonance, mass spectrometry, and chromatography profiles with those of cineole standards. Streptomyces griseus yielded 8-hydroxy-1,4-cineole as the major hydroxylation product together with 2-exo- and 2-endo-hydroxy-1,4-cineoles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. R., Hoffmann H. M., Kieslich K., Reng G., Stumpf B. Microbial transformations of some monoterpenoids and sesquiterpenoids. Ciba Found Symp. 1985;111:146–160. doi: 10.1002/9780470720929.ch11. [DOI] [PubMed] [Google Scholar]
- Betts R. E., Walters D. E., Rosazza J. P. Microbial transformations of antitumor compounds. 1. Conversion of acronycine to 9-hydroxyacronycine by Cunninghamella echinulata. J Med Chem. 1974 Jun;17(6):599–602. doi: 10.1021/jm00252a006. [DOI] [PubMed] [Google Scholar]



