Abstract
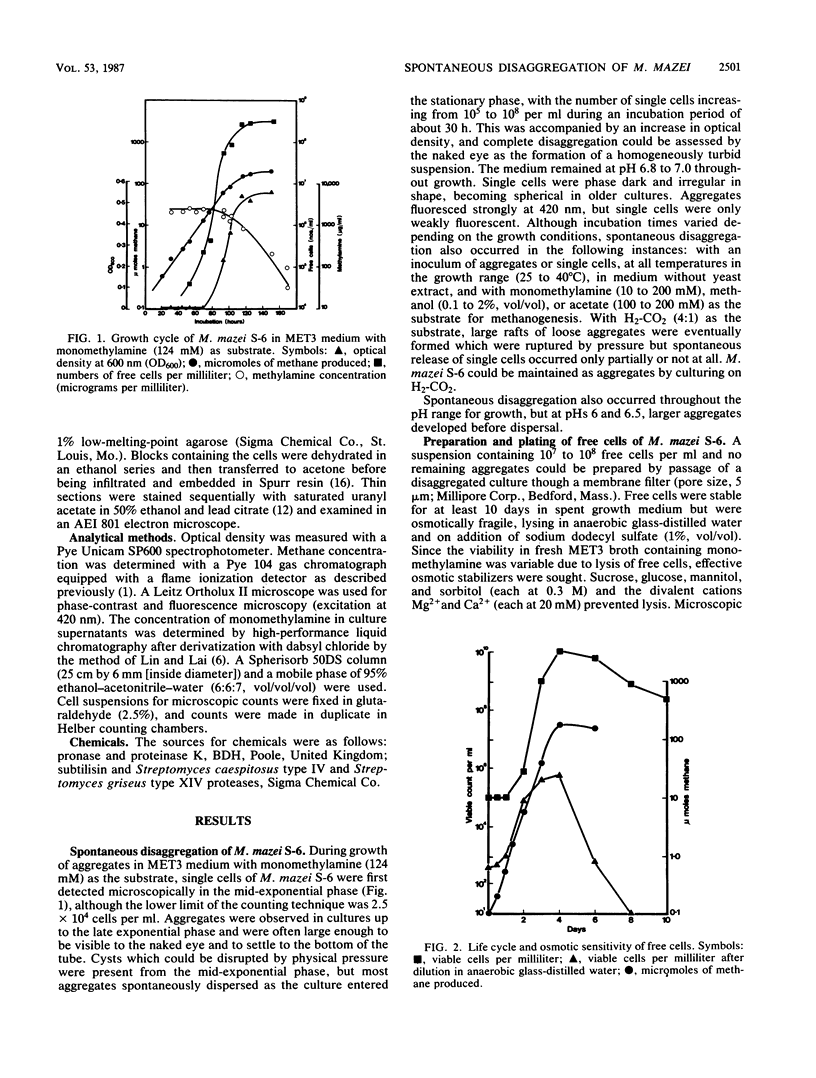
When monomethylamine was the growth substrate, spontaneous disaggregation of Methanosarcina mazei S-6 commenced at the mid-exponential phase and resulted in the formation of a suspension containing 108 to 109 free cells per ml. Free cells were osmotically fragile and amenable to extraction of DNA. Hypertonic media for the manipulation and regeneration of free cells into aggregates were developed, and plating efficiencies of 100% were achieved for M. mazei S-6 and LYC. Free cells of strain S-6 required MgCl2 (10 mM) for growth, whereas aggregates did not. Specific growth rates of strains S-6 and LYC were increased by MgCl2. Treatment with pronase caused sphere formation and removal of the protein wall of cells of strain S-6, but protoplasts could not be regenerated. The disaggregating enzyme produced by strain S-6 facilitated the preparation of suspensions of free cells of some strains of Methanosarcina barkeri. Although this provided a means of extracting high-molecular-weight DNA from M. barkeri, less than 0.1% of free cells were viable.
Full text
PDF
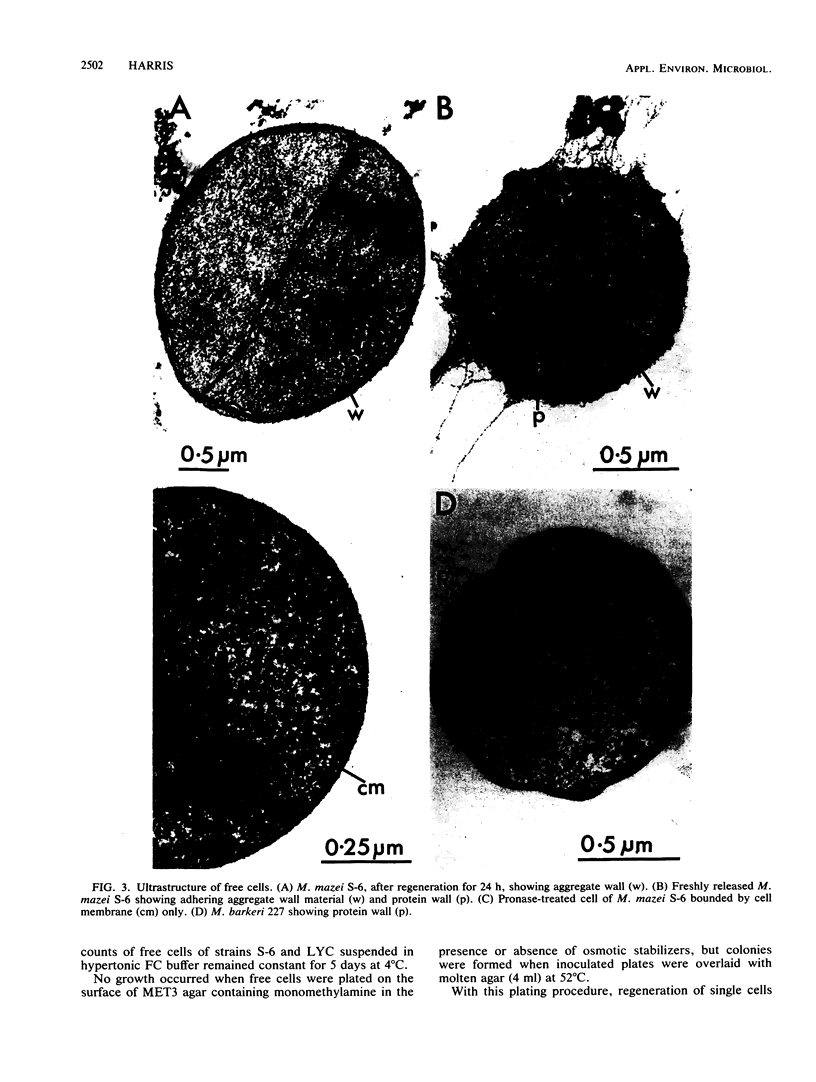

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
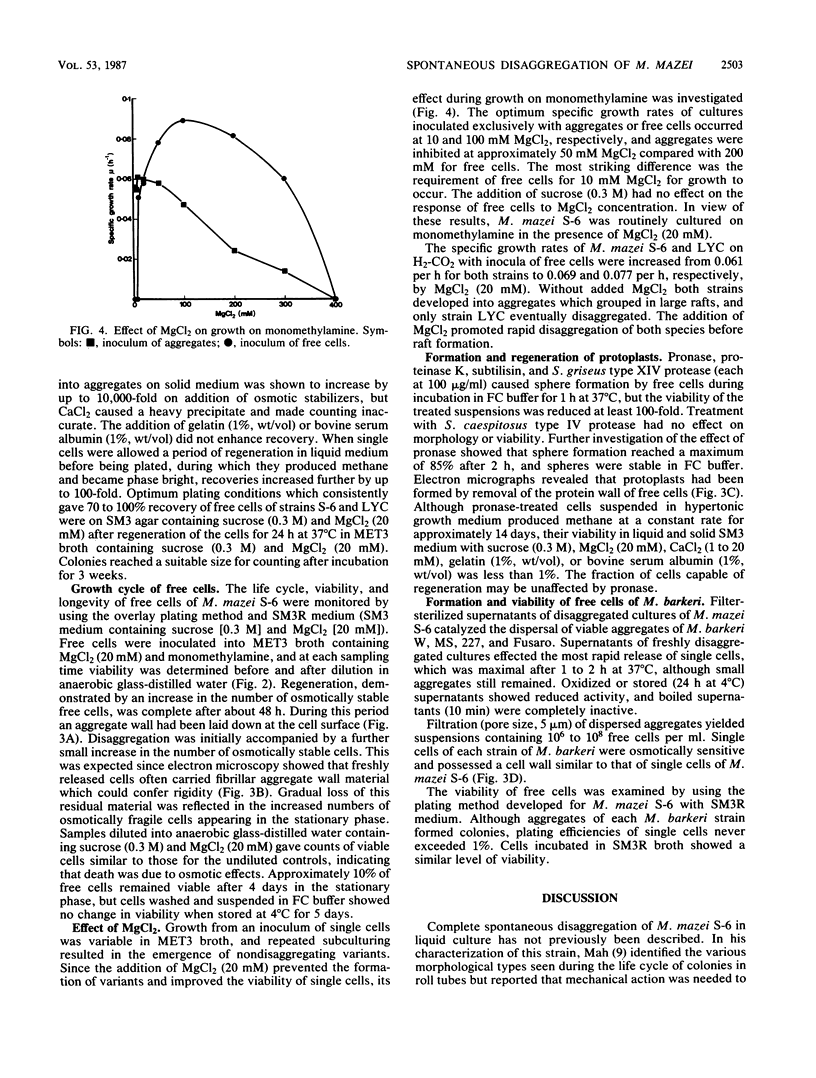
- Kandler O., Hippe H. Lack of peptidoglycan in the cell walls of Methanosarcina barkeri. Arch Microbiol. 1977 May 13;113(1-2):57–60. doi: 10.1007/BF00428580. [DOI] [PubMed] [Google Scholar]
- Lin J. K., Lai C. C. High performance liquid chromatographic determination of naturally occurring primary and secondary amines with dabsyl chloride. Anal Chem. 1980 Apr;52(4):630–635. doi: 10.1021/ac50054a008. [DOI] [PubMed] [Google Scholar]
- Liu Y., Boone D. R., Sleat R., Mah R. A. Methanosarcina mazei LYC, a New Methanogenic Isolate Which Produces a Disaggregating Enzyme. Appl Environ Microbiol. 1985 Mar;49(3):608–613. doi: 10.1128/aem.49.3.608-613.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. W. Life Cycles in the Methanogenic Archaebacterium Methanosarcina mazei. Appl Environ Microbiol. 1986 Jul;52(1):17–27. doi: 10.1128/aem.52.1.17-27.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Baron S. F., Ferry J. G. Methanosarcina acetivorans sp. nov., an Acetotrophic Methane-Producing Bacterium Isolated from Marine Sediments. Appl Environ Microbiol. 1984 May;47(5):971–978. doi: 10.1128/aem.47.5.971-978.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Ferry J. G. Isolation and Characterization of a Methylotrophic Marine Methanogen, Methanococcoides methylutens gen. nov., sp. nov. Appl Environ Microbiol. 1983 Feb;45(2):684–690. doi: 10.1128/aem.45.2.684-690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]