Abstract
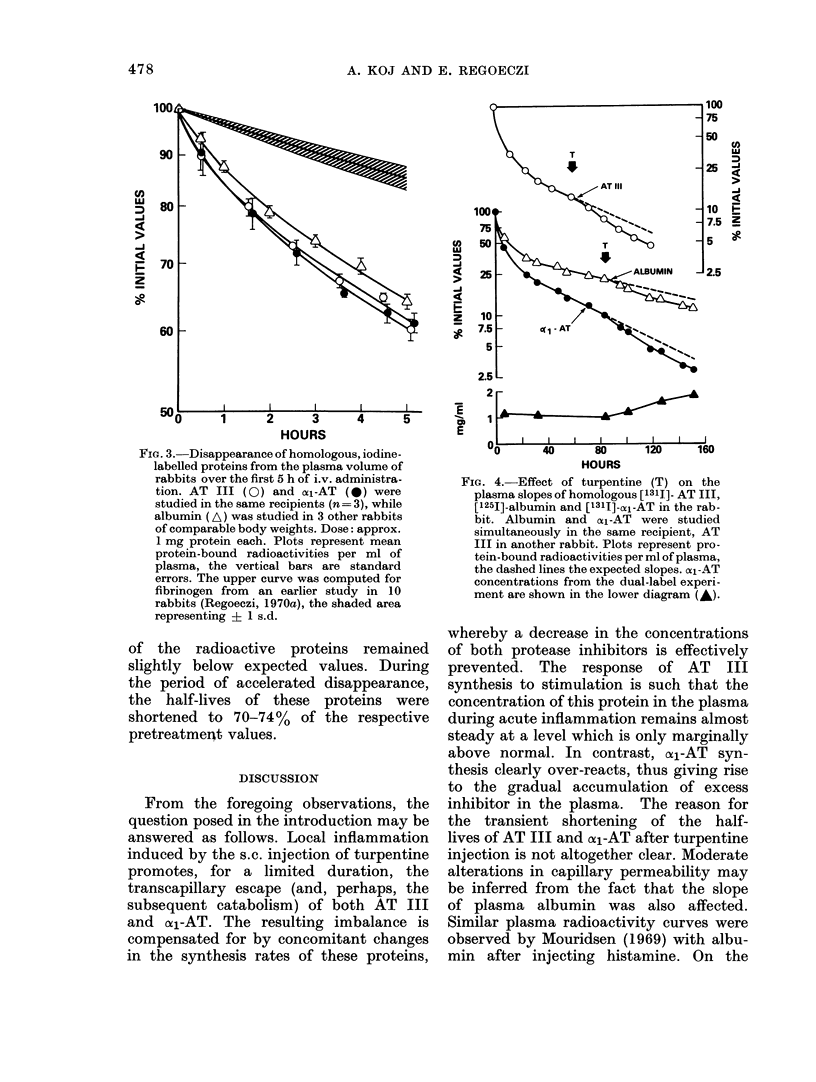
Local inflammation, induced by s.c. injection of turpentine, evoked characteristic changes in the metabolism of antithrombin III, and alpha1-antitrypsin. For a period of approximately 36 h, the plasma half-lives of both protease inhibitors were shortened to 70--74% of the respective preinjection values. Similar changes were also observed in the slope of iodine-labelled albumin, suggesting that increased capillary permeability was primarily responsible for the losses of labelled proteins from the circulation. Incorporation of [3H]- or [14C]-leucine into albumin changed little during inflammation, but markedly increased values were measured for anti-thrombin III (3-fold), alpha1-antitrypsin (4-fold) and, above all, for fibrinogen (7-fold) 24 h and 48 h after the injection of turpentine. These changes in synthesis and elimination rates resulted in the following net balances: fibrinogen concentrations in plasma rose substantially during the early phase of inflammation; alpha1-antitrypsin concentrations increased gradually but to a significantly lesser extent, peak concentrations being reached after a reverse trend in fibrinogen concentrations had become apparent; antithrombin III concentrations remained steady throughout at levels which were only marginally above the pretreatment values.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atencio A. C., Chao P. Y., Chen A. Y., Reeve E. B. Fibrinogen response to corticotropin preparations in rabbits. Am J Physiol. 1969 Apr;216(4):773–780. doi: 10.1152/ajplegacy.1969.216.4.773. [DOI] [PubMed] [Google Scholar]
- Atencio A. C., Joiner K., Reeve E. B. Experimental and control systems studies of plasma fibrinogen regulation in rabbits. Am J Physiol. 1969 Apr;216(4):764–772. doi: 10.1152/ajplegacy.1969.216.4.764. [DOI] [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- EGEBERG O. INHERITED ANTITHROMBIN DEFICIENCY CAUSING THROMBOPHILIA. Thromb Diath Haemorrh. 1965 Jun 15;13:516–530. [PubMed] [Google Scholar]
- Gordon A. H., Koj A. Changes in the rates of synthesis of certain plasma proteins following tissue damage due to talc injection. A study using the perfused rat liver. Br J Exp Pathol. 1968 Oct;49(5):436–447. [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Human granulocyte elastase. Further delineation of its role in connective tissue damage. Am J Pathol. 1972 Sep;68(3):579–592. [PMC free article] [PubMed] [Google Scholar]
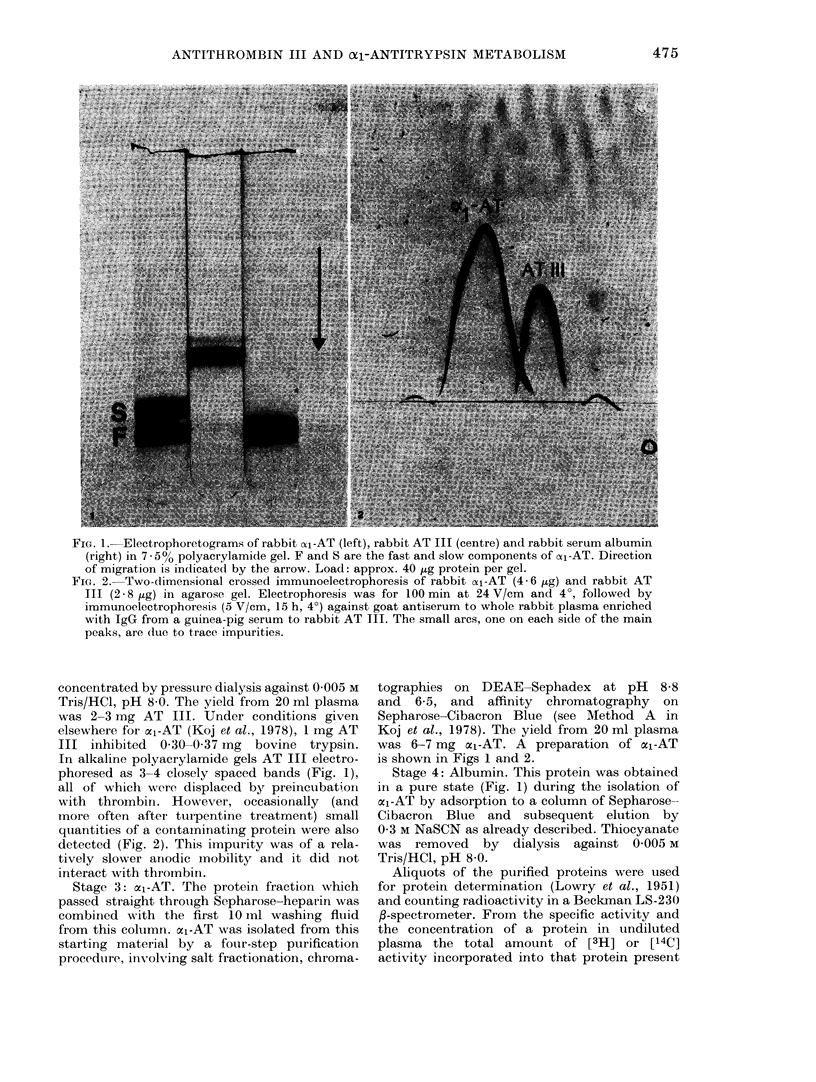
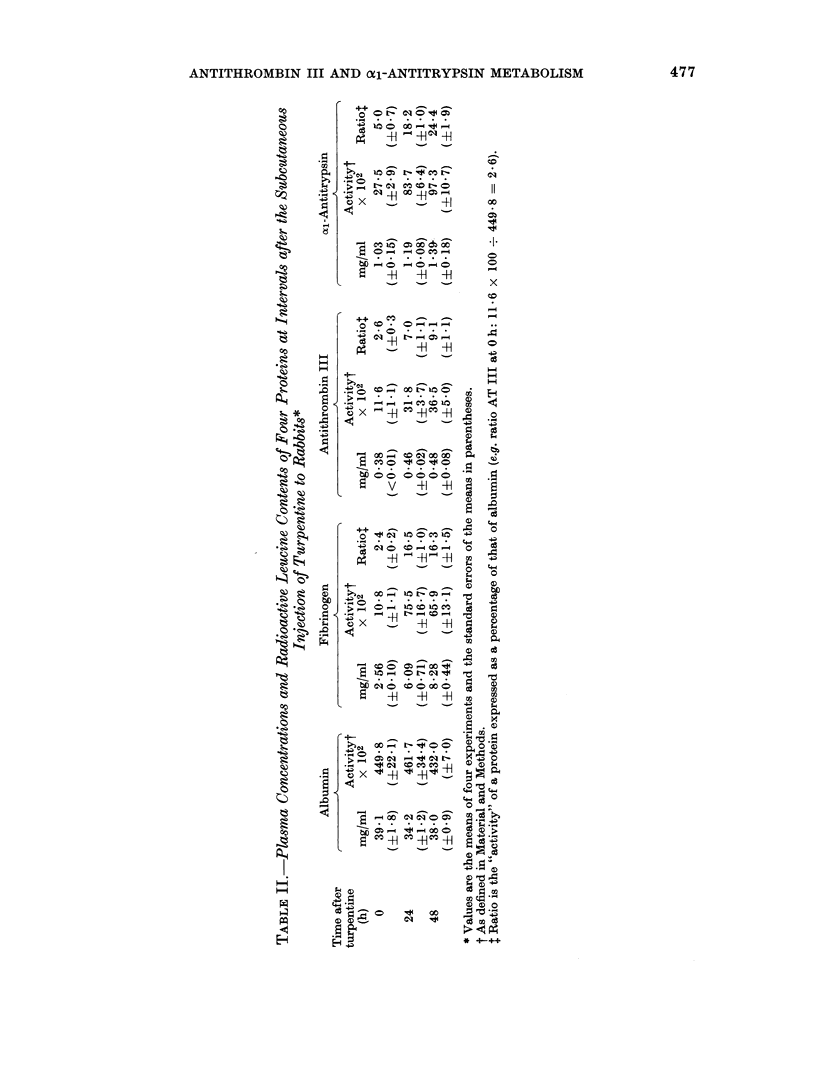
- Koj A., Hatton M. W., Wong K. L., Regoeczi E. Isolation and partial characterization of rabbit plasma alpha1-antitrypsin. Biochem J. 1978 Mar 1;169(3):589–596. doi: 10.1042/bj1690589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koj A., McFarlane A. S. Effect of endotoxin on plasma albumin and fibrinogen synthesis rates in rabbits as measured by the [14C] carbonate method. Biochem J. 1968 Jun;108(1):137–146. doi: 10.1042/bj1080137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koj A. The measurement of absolute and relative synthesis rates of fibrinogen in vivo. Biochim Biophys Acta. 1968 Aug 6;165(1):97–107. doi: 10.1016/0304-4165(68)90193-1. [DOI] [PubMed] [Google Scholar]
- Kueppers F. Alpha1-antitrypsin. Am J Hum Genet. 1973 Nov;25(6):677–686. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marciniak E., Farley C. H., DeSimone P. A. Familial thrombosis due to antithrombin 3 deficiency. Blood. 1974 Feb;43(2):219–231. [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Mouridsen H. T. The extravascular retention of albumin in wound tissue and its contribution to the postoperative hypoalbuminaemia in rabbits. Clin Sci. 1969 Oct;37(2):431–441. [PubMed] [Google Scholar]
- Ohlsson K. Alpha1-antitrypsin and alpha2-macroglobulin. Interactions with human neutrophil collagenase and elastase. Ann N Y Acad Sci. 1975 Jun 13;256:409–419. doi: 10.1111/j.1749-6632.1975.tb36067.x. [DOI] [PubMed] [Google Scholar]
- REEVE E. B., PEARSON J. R., MARTZ D. C. Plasma protein synthesis in the liver: method for measurement of albumin formation in vivo. Science. 1963 Mar 8;139(3558):914–916. doi: 10.1126/science.139.3558.914. [DOI] [PubMed] [Google Scholar]
- Regoeczi E. Fibrinogen catabolism: kinetics of catabolism following sudden elevation of the pool with exogenous fibrinogen. Clin Sci. 1970 Jan;38(1):111–121. doi: 10.1042/cs0380111. [DOI] [PubMed] [Google Scholar]
- Regoeczi E., Hatton M. W., Long K. L. Studies of the metabolism of asialotransferrins: potentiation of the catabolism of human asialotransferrin in the rabbit. Can J Biochem. 1974 Mar;52(3):155–161. doi: 10.1139/o74-026. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D. Chemistry of the hemostatic mechanism and its relationship to the action of heparin. Fed Proc. 1977 Jan;36(1):10–18. [PubMed] [Google Scholar]
- Sas G., Blaskó G., Bánhegyi D., Jákó J., Pálos L. A. Abnormal antithrombin III (antithrombin III "Budapest") as a cause of a familial thrombophilia. Thromb Diath Haemorrh. 1974 Sep 30;32(1):105–115. [PubMed] [Google Scholar]
- Travis J., Bowen J., Tewksbury D., Johnson D., Pannell R. Isolation of albumin from whole human plasma and fractionation of albumin-depleted plasma. Biochem J. 1976 Aug 1;157(2):301–306. doi: 10.1042/bj1570301. [DOI] [PMC free article] [PubMed] [Google Scholar]