Abstract
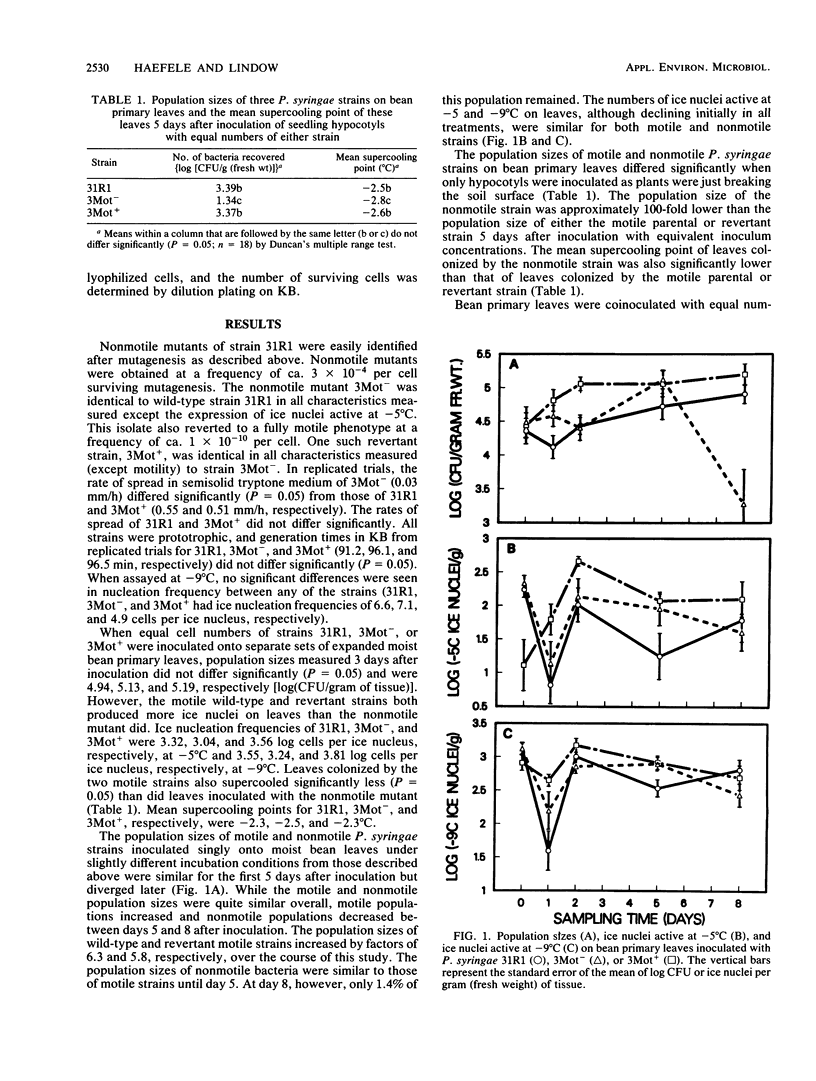
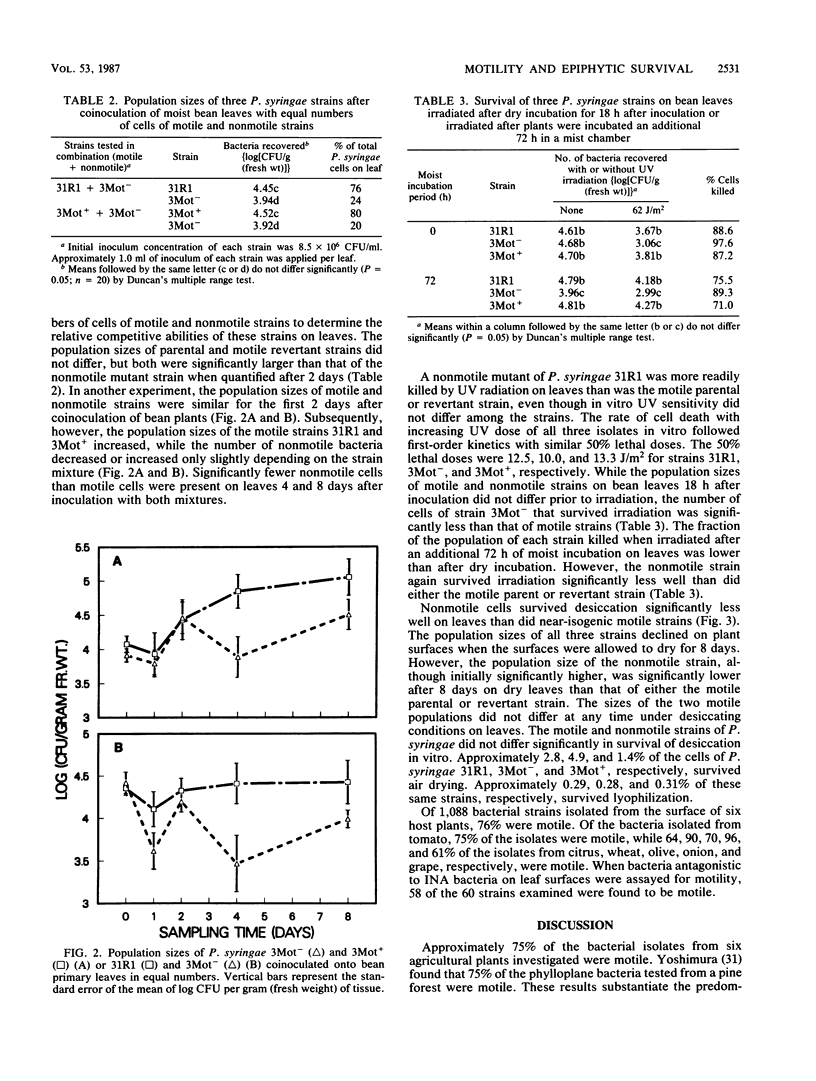
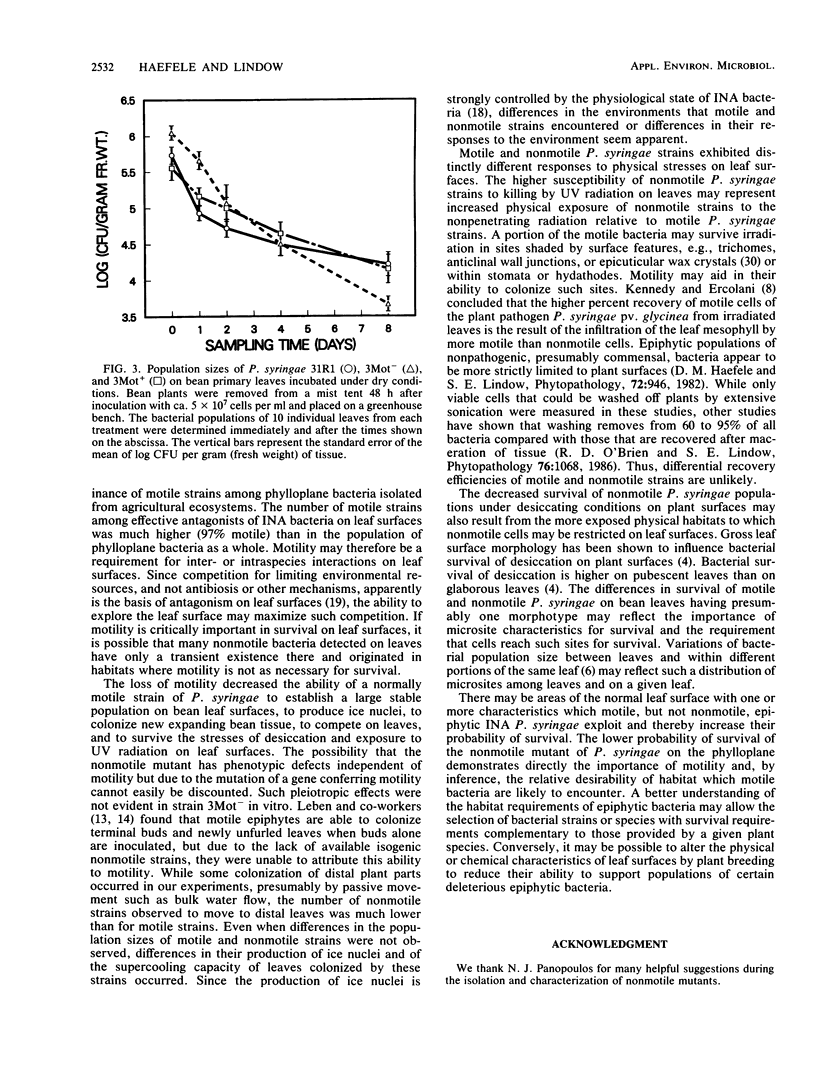
The role of flagellar motility in determining the epiphytic fitness of an ice-nucleation-active strain of Pseudomonas syringae was examined. The loss of flagellar motility reduced the epiphytic fitness of a normally motile P. syringae strain as measured by its growth, survival, and competitive ability on bean leaf surfaces. Equal population sizes of motile parental or nonmotile mutant P. syringae strains were maintained on bean plants for at least 5 days following the inoculation of fully expanded primary leaves. However, when bean seedlings were inoculated before the primary leaves had expanded and bacterial populations on these leaves were quantified at full expansion, the population size of the nonmotile derivative strain reached only 0.9% that of either the motile parental or revertant strain. When fully expanded bean primary leaves were coinoculated with equal numbers of motile and nonmotile cells, the population size of a nonmotile derivative strain was one-third of that of the motile parental or revertant strain after 8 days. Motile and nonmotile cells were exposed in vitro and on plants to UV radiation and desiccating conditions. The motile and nonmotile strains exhibited equal resistance to both stresses in vitro. However, the population size of a nonmotile strain on leaves was less than 20% that of a motile revertant strain when sampled immediately after UV irradiation. Epiphytic populations of both motile and nonmotile P. syringae declined under desiccating conditions on plants, and after 8 days, the population size of a nonmotile strain was less than one-third that of the motile parental or revertant strain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S. S., Baker L. S., Upper C. D. Ice nucleation temperature of individual leaves in relation to population sizes of ice nucleation active bacteria and frost injury. Plant Physiol. 1985 Feb;77(2):259–265. doi: 10.1104/pp.77.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kelman A., Hruschka J. The role of motility and aerotaxis in the selective increase of avirulent bacteria in still broth cultures of Pseudomonas solanacearum. J Gen Microbiol. 1973 May;76(1):177–188. doi: 10.1099/00221287-76-1-177. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Leben C., Daft G. C. Migration of bacteria on seedling plants. Can J Microbiol. 1966 Dec;12(6):1119–1123. doi: 10.1139/m66-153. [DOI] [PubMed] [Google Scholar]
- Leben C. Influence of humidity on the migration of bacteria on cucumber seedlings. Can J Microbiol. 1965 Aug;11(4):671–676. doi: 10.1139/m65-090. [DOI] [PubMed] [Google Scholar]
- Leben C., Whitmoyer R. E. Adherence of bacteria to leaves. Can J Microbiol. 1979 Aug;25(8):896–901. doi: 10.1139/m79-133. [DOI] [PubMed] [Google Scholar]
- Maki L. R., Galyan E. L., Chang-Chien M. M., Caldwell D. R. Ice nucleation induced by pseudomonas syringae. Appl Microbiol. 1974 Sep;28(3):456–459. doi: 10.1128/am.28.3.456-459.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Doetsch R. N. Motility in Pseudomonas fluorescens with special reference to survival advantage and negative chemotaxis. Life Sci. 1968 Aug 15;7(16):875–886. doi: 10.1016/0024-3205(68)90119-7. [DOI] [PubMed] [Google Scholar]



