Abstract
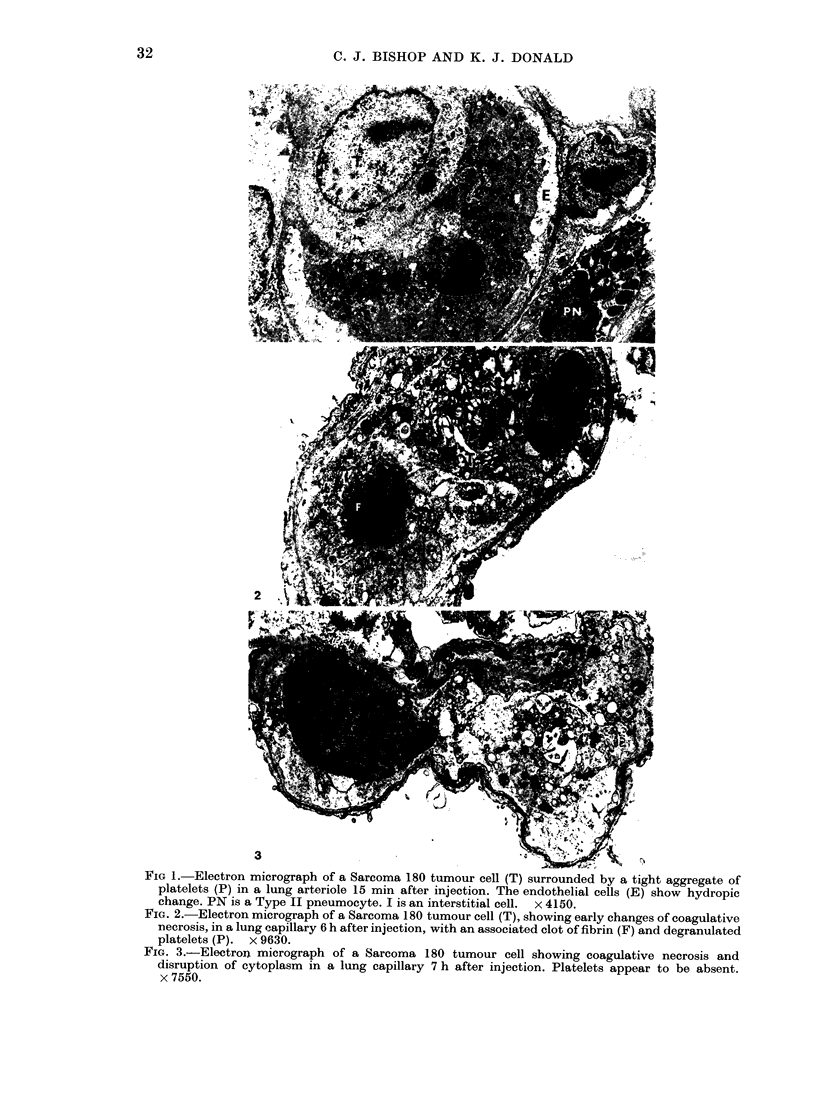
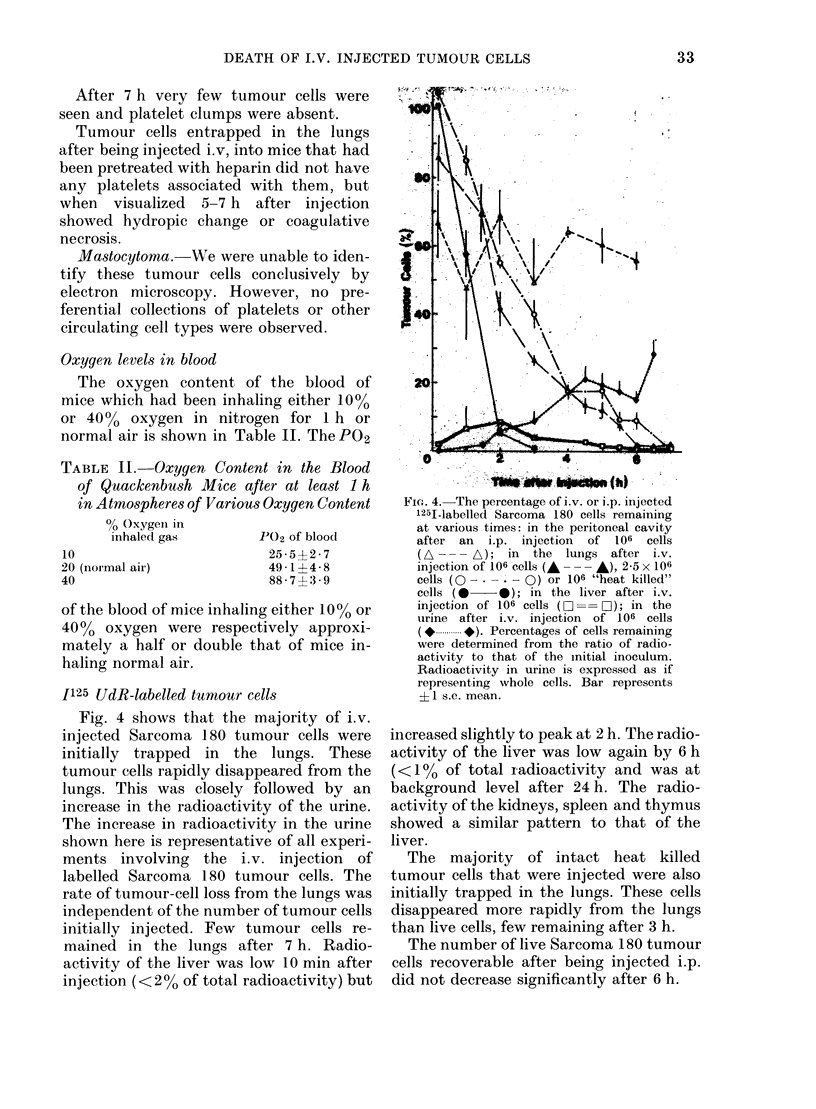
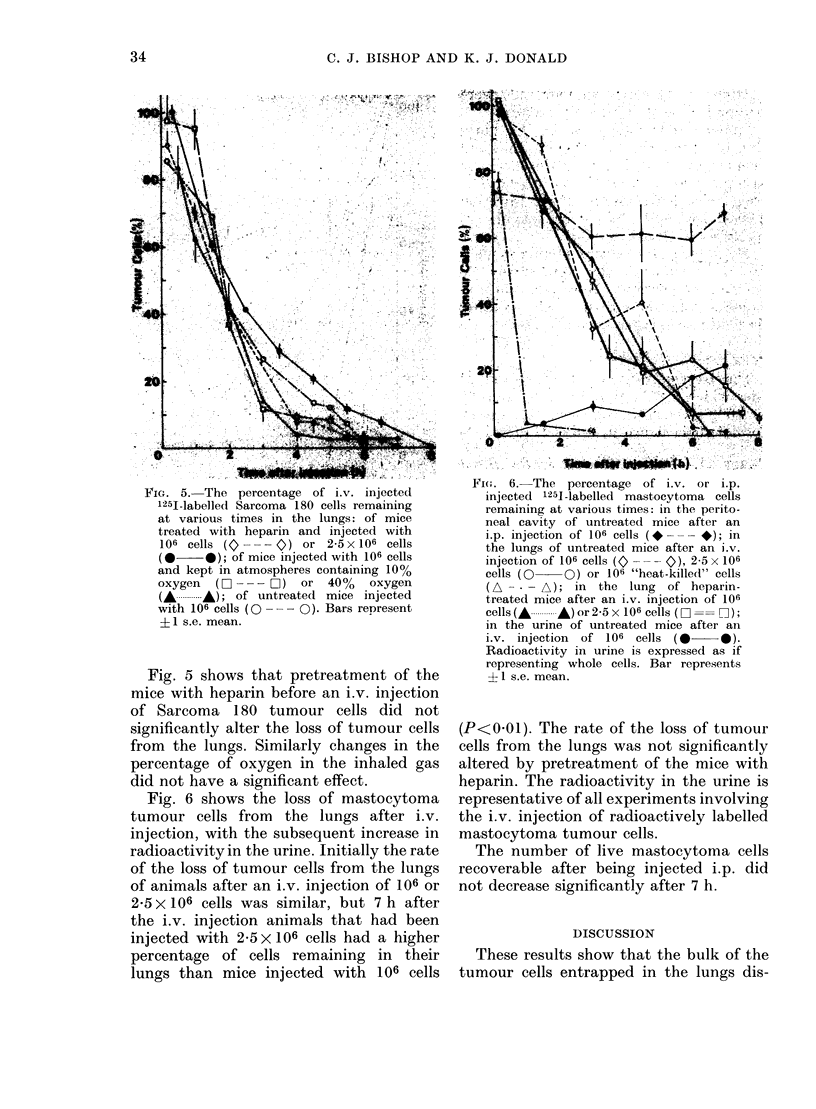
Most DBA mastocytoma and Sarcoma 180 cells trapped in the lungs of mice after i.v. injection died within 7 h. Rates of cell death were similar for both tumour cell lines. Rates of tumour cell death were unrelated to whether the cells were allogeneic or syngeneic, induced platelet aggregation or not, had different patterns of subsequent tumour growth, or were injected in varying numbers. Cell death was by coagulative necrosis, not apoptosis. Sarcoma 180 tumour cells were quickly localized in the lung and enclosed in platelet aggregates which remained, with degranulation, until the time of tumour cell death. However, platelet aggregation did not appear to play a role in tumour cell killing. The prevention of platelet aggregation by pretreatment of mice with an anticoagulant had little effect on the rate of death of tumour cells in the lung. Mastocytoma tumour cells did not cause platelet aggregation, yet died in the lung at similar rates to Sarcoma 180 cells. The killing of tumour cells in the lung did not appear to be cell-mediated. No mononuclear cells were seen in the vicinity of tumour cells and the type of cell death was not that associated with cell-mediated killing. The tumour cells did not die within 6 h of being injected into the peritoneal cavity. It is suggested that a nonspecific non-immunological process results in the death of intravenously injected tumour cells in the lung. This process was not affected by differing oxygen levels in the inhaled gas.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablett G., Bishop C., Sheridan J. W., Donald K. J. Inhibition of the growth of murine tumour cells in vitro by serum from non-immune syngeneic and allogeneic mice. Br J Exp Pathol. 1978 Oct;59(5):522–529. [PMC free article] [PubMed] [Google Scholar]
- BASERGA R., KISIELESKI W. E., HALVORSEN K. A study on the establishment and growth of tumor metastases with tritiated thymidine. Cancer Res. 1960 Jul;20:910–917. [PubMed] [Google Scholar]
- Brown J. M. A study of the mechanism by which anticoagulation with warfarin inhibits blood-borne metastases. Cancer Res. 1973 Jun;33(6):1217–1224. [PubMed] [Google Scholar]
- Chew E. C., Wallace A. C. Demonstration of fibrin in early stages of experimental metastases. Cancer Res. 1976 Jun;36(6):1904–1909. [PubMed] [Google Scholar]
- Don M. M., Ablett G., Bishop C. J., Bundesen P. G., Donald K. J., Searle J., Kerr J. F. Death of cells by apoptosis following attachment of specifically allergized lymphocytes in vitro. Aust J Exp Biol Med Sci. 1977 Aug;55(4):407–417. doi: 10.1038/icb.1977.38. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970 Oct;45(4):773–782. [PubMed] [Google Scholar]
- Fisher B., Fisher E. R. Anticoagulants and tumor cell lodgement. Cancer Res. 1967 Feb;27(2):421–425. [PubMed] [Google Scholar]
- Fisher B., Fisher E. R. The organ distribution of disseminated 51 Cr-labeled tumor cells. Cancer Res. 1967 Feb;27(2):412–420. [PubMed] [Google Scholar]
- GREENE H. S., HARVEY E. K. THE RELATIONSHIP BETWEEN THE DISSEMINATION OF TUMOR CELLS AND THE DISTRIBUTION OF METASTASES. Cancer Res. 1964 Jun;24:799–811. [PubMed] [Google Scholar]
- Gershon R. K., Carter R. L., Kondo K. On concomitant immunity in tumour-bearing hamsters. Nature. 1967 Feb 18;213(5077):674–676. doi: 10.1038/213674a0. [DOI] [PubMed] [Google Scholar]
- Hilgard P., Beyerle L., Hohage R., Hiemeyer V., Kübler M. The effect of heparin on the initial phase of metastasis formation. Eur J Cancer. 1972 Jun;8(3):347–352. doi: 10.1016/0014-2964(72)90031-x. [DOI] [PubMed] [Google Scholar]
- Hofer K. G., Prensky W., Hughes W. L. Death and metastatic distribution of tumor cells in mice monitored with 125I-iododeoxy-uridine. J Natl Cancer Inst. 1969 Oct;43(4):763–773. [PubMed] [Google Scholar]
- Jones D. S., Wallace A. C., Fraser E. E. Sequence of events in experimental metastases of Walker 256 tumor: light, immunofluorescent, and electron microscopic observations. J Natl Cancer Inst. 1971 Mar;46(3):493–504. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Petranyi G., Kärre K., Jondal M., Tracey D., Wigzell H. Killer cells: a functional comparison between natural, immune T-cell and antibody-dependent in vitro systems. J Exp Med. 1976 Apr 1;143(4):772–780. doi: 10.1084/jem.143.4.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J., Goldblatt P. J., Leighton J. Ultrastructural features of invasion in chick embryo liver metastasis of Yoshida ascites hepatoma. Cancer Res. 1970 Jun;30(6):1632–1644. [PubMed] [Google Scholar]
- Martin S. E., Martin W. J. Anti-tumour antibodies in normal mouse sera. Int J Cancer. 1975 Apr 15;15(4):658–664. doi: 10.1002/ijc.2910150415. [DOI] [PubMed] [Google Scholar]
- Ménard S., Colnaghi M. I., Porta G. D. Natural anti-tumor serum reactivity in BALB/c mice. I. Characterization and interference with tumor growth. Int J Cancer. 1977 Feb 15;19(2):267–274. doi: 10.1002/ijc.2910190217. [DOI] [PubMed] [Google Scholar]
- Proctor J. W., Auclair B. G., Rudenstam C. M. The distribution and fate of blood-borne 125IUdR-labelled tumour cells in immune syngeneic rats. Int J Cancer. 1976 Aug 15;18(2):255–262. doi: 10.1002/ijc.2910180217. [DOI] [PubMed] [Google Scholar]
- Rannie G. H., Donald K. J. Estimation of the migration of thoracic duct lymphocytes to non-lymphoid tissues. A comparison of the distribution of radioactivity at intervals following i.v. transfusion of cells labelled with 3H, 14C, 75Se, 99mTc, 125I and 51Cr in the rat. Cell Tissue Kinet. 1977 Nov;10(6):523–541. [PubMed] [Google Scholar]
- Russell S. W., Rosenau W., Lee J. C. Cytolysis induced by human lymphotoxin. Am J Pathol. 1972 Oct;69(1):103–118. [PMC free article] [PubMed] [Google Scholar]
- Sadler T. E., Alexander P. Trapping and destruction of blood-borne syngeneic leukaemia cells in lung, liver and spleen of normal and leukaemic rats. Br J Cancer. 1976 May;33(5):512–520. doi: 10.1038/bjc.1976.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Glauert A. M. The mechanism of T cell mediated cytotoxicity. V. Morphological studies by electron microscopy. Proc R Soc Lond B Biol Sci. 1977 Sep 5;198(1132):315–323. doi: 10.1098/rspb.1977.0100. [DOI] [PubMed] [Google Scholar]
- Schiffer L. M., Nelson J. S., Dilettuso B., Migliorato D., Randolph W. Cytokinetics of the S-180 ascites tumor system. Cell Tissue Kinet. 1973 Mar;6(2):165–172. doi: 10.1111/j.1365-2184.1973.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Van Den Brenk H. A., Burch W. M., Kelly H., Orton C. Venous diversion trapping and growth of blood-borne cancer cells en route to the lungs. Br J Cancer. 1975 Jan;31(1):46–61. doi: 10.1038/bjc.1975.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaeminck M. N., Adenis L., Mouton Y., Demaille A. Etude expérimentale de la diffusion métastatique chez l'oeuf de poule embryonné. Répartition, microscopie et ultrastructure des foyers tumoraux. Int J Cancer. 1972 Nov;10(3):619–631. doi: 10.1002/ijc.2910100322. [DOI] [PubMed] [Google Scholar]
- ZEIDMAN I., McCUTCHEON M., COMAN D. R. Factors affecting the number of tumor metastases; experiments with a transplantable mouse tumor. Cancer Res. 1950 Jun;10(6):357–359. [PubMed] [Google Scholar]
- Zuckerberg C. Ultrastructure of sarcoma 180. Cancer Res. 1973 Oct;33(10):2278–2282. [PubMed] [Google Scholar]