Abstract
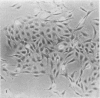
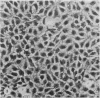
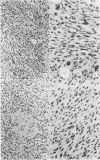
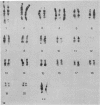
Two types of tumour cell have been obtained from a glioma transplacentally induced by ethylnitrosourea in a BD-IX rat. These were distinguished in culture by their different morphologies, responses to dibutyryl cyclic adenosine monophosphate, inducibility of glycerol phosphate dehydrogenase and growth in soft agar. They had different karyotypes, with distinctive numbers and arrangements of chromosomes. One cell type had an apparently normal diploid set of 42 whilst the other had 43 chromosomes. An additional chromosome No 4 was identified in the latter by Giemsa banding. Translocations and other abnormalities involving this chromosome were consistently observed. Both cell types produced malignant, astrocytic tumours when injected into newborn syngeneic rats, but with different latent periods and morphological features.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au W., Soukup S. W., Mandybur T. I. Excess chromosome no. 4 in ethylnitrosourea-induced neurogenic tumor lines of the rat. J Natl Cancer Inst. 1977 Dec;59(6):1709–1716. doi: 10.1093/jnci/59.6.1709. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Rucker N., Mark C., Kouri R. E. Correlation between balance of specific chromosomes and expression of malignancy in hamster cells. J Natl Cancer Inst. 1975 Jan;54(1):157–162. doi: 10.1093/jnci/54.1.157. [DOI] [PubMed] [Google Scholar]
- Claisse P. J., Lantos P. L., Roscoe J. P. Analysis of N-ethyl-N-nitrosourea-induced brain carcinogenesis by sequential culturing during the latent period. II. Morphology of the tumors induced by cell cultures. J Natl Cancer Inst. 1978 Aug;61(2):391–398. [PubMed] [Google Scholar]
- Claisse P. J., Roscoe J. P. The inducibility of glycerol phosphate dehydrogenase in two rat glial clones. Brain Res. 1976 Jun 11;109(2):423–425. doi: 10.1016/0006-8993(76)90547-3. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. The origin and development of human tumors studied with cell markers. N Engl J Med. 1974 Jul 4;291(1):26–35. doi: 10.1056/NEJM197407042910109. [DOI] [PubMed] [Google Scholar]
- Fraccaro M., Mannini A., Tiepolo L., Gerli M., Zara C. Karyotyping clonal evolution in a cystic adenoma of the ovary. Lancet. 1968 Mar 23;1(7543):613–614. doi: 10.1016/s0140-6736(68)91235-x. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., Richardson C. R. An improved banding technique exemplified in the karyotype analysis of two strains of rat. Chromosoma. 1973;41(3):259–263. doi: 10.1007/BF00344020. [DOI] [PubMed] [Google Scholar]
- HENDERSON J. S., ROUS P. The plating of tumor components on the subcutaneous expanses of young mice. Findings with benign and malignant epidermal growths and with mammary carcinomas. J Exp Med. 1962 Jun 1;115:1211–1230. doi: 10.1084/jem.115.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hince T. A., Roscoe J. P. Fibrinolytic activity of cultured cells derived during ethylnitrosourea-induced carcinogenesis of rat brain. Br J Cancer. 1978 Mar;37(3):424–433. doi: 10.1038/bjc.1978.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone P. M., Gardner R. L., Harris H. The cellular origin of chemically induced tumours. J Cell Sci. 1978 Feb;29:249–269. doi: 10.1242/jcs.29.1.249. [DOI] [PubMed] [Google Scholar]
- Levan G., Ahlström U., Mitelman F. The specificity of chromosome A2 involvement in DMBA-induced rat sarcomas. Hereditas. 1974;77(2):263–280. doi: 10.1111/j.1601-5223.1974.tb00939.x. [DOI] [PubMed] [Google Scholar]
- MAKINO S. Further evidence favoring the concept of the stem cell in ascites tumors of rats. Ann N Y Acad Sci. 1956 Mar 14;63(5):818–830. doi: 10.1111/j.1749-6632.1956.tb50894.x. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Roscoe J. P., Claisse P. J. A sequential in vivo-in vitro study of carcinogenesis induced in the rat brain by ethylnitrosourea. Nature. 1976 Jul 22;262(5566):314–316. doi: 10.1038/262314a0. [DOI] [PubMed] [Google Scholar]
- Roscoe J. P., Claisse P. J. Analysis of N-ethyl-N-nitrosourea-induced brain carcinogenesis by sequential culturing during the latent period. I. Morphology and tumorigenicity of the cultured cells and their growth in agar. J Natl Cancer Inst. 1978 Aug;61(2):381–390. [PubMed] [Google Scholar]
- Roscoe J. P., Gibbs B. E. Several changes associated with the acquisition of a single chromosome in rat glial tumor cells. J Natl Cancer Inst. 1974 Aug;53(2):581–583. doi: 10.1093/jnci/53.2.581. [DOI] [PubMed] [Google Scholar]
- SCHRECKER A. W., VENDITTI J. M., GREENBERG N. H., BIEDLER J. L., ROBINSON D. L., HUTCHISON D. J. ASSOCIATION OF INCREASED DIHYDROFOLATE REDUCTASE LEVELS AND CHROMOSOME ALTERATION IN AMETHOPTERIN-RESISTANT SUBLINES OF LEUKEMIA L1210. J Natl Cancer Inst. 1963 Sep;31:557–574. [PubMed] [Google Scholar]
- Sandberg A. A., Hossfeld D. K. Chromosomal abnormalities in human neoplasia. Annu Rev Med. 1970;21:379–408. doi: 10.1146/annurev.me.21.020170.002115. [DOI] [PubMed] [Google Scholar]
- Uenaka H., Ueda N., Maeda S., Sugiyama T. Involvement of chromosome No. 2 in chromosome changes in primary leukemia induced in rats by N-nitroso-N-butylurea. J Natl Cancer Inst. 1978 Jun;60(6):1399–1404. doi: 10.1093/jnci/60.6.1399. [DOI] [PubMed] [Google Scholar]
- Winslow D. P., Roscoe J. P., Rowles P. M. Changes in surface morphology associated with ethylnitrosourea-induced malignant transformation of cultured rat brain cells studied by scanning electron microscopy. Br J Exp Pathol. 1978 Oct;59(5):530–539. [PMC free article] [PubMed] [Google Scholar]
- al-Saadi A., Beierwaltes W. H. Sequential cytogenetic changes in the evolution of transplanted thyroid tumors to metastatic carcinoma in the Fischer rat. Cancer Res. 1967 Oct;27(10):1831–1842. [PubMed] [Google Scholar]