Abstract
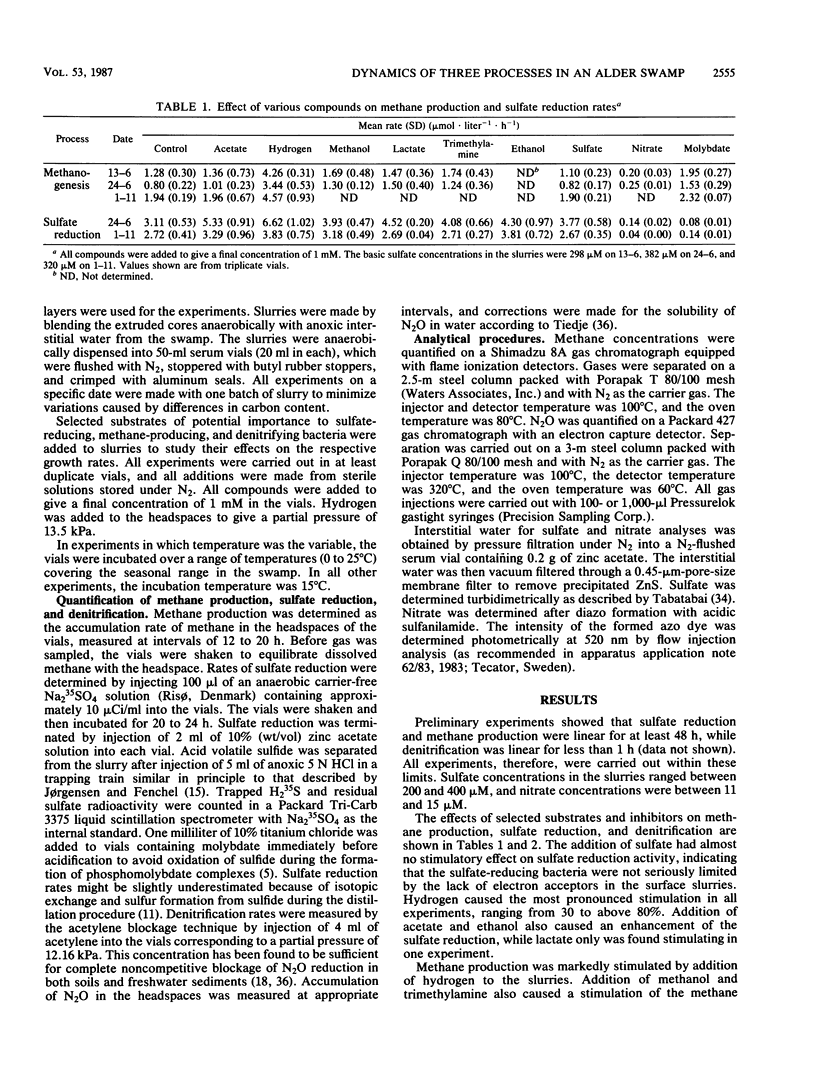
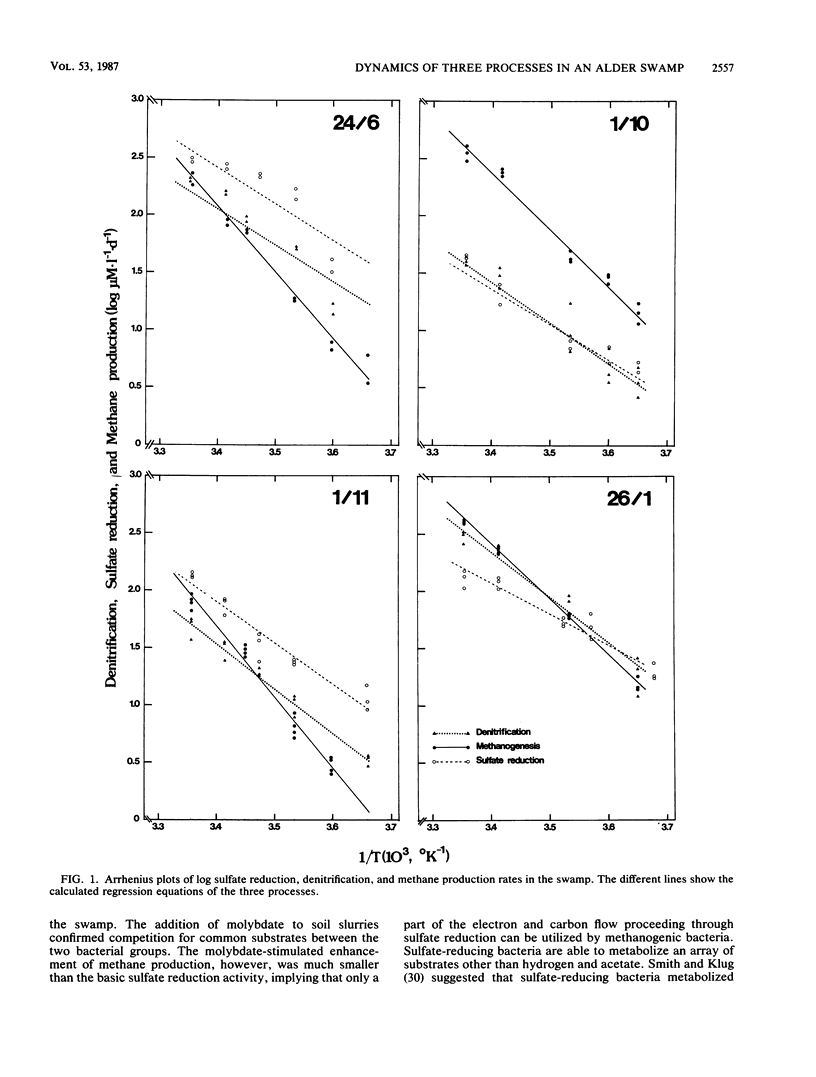
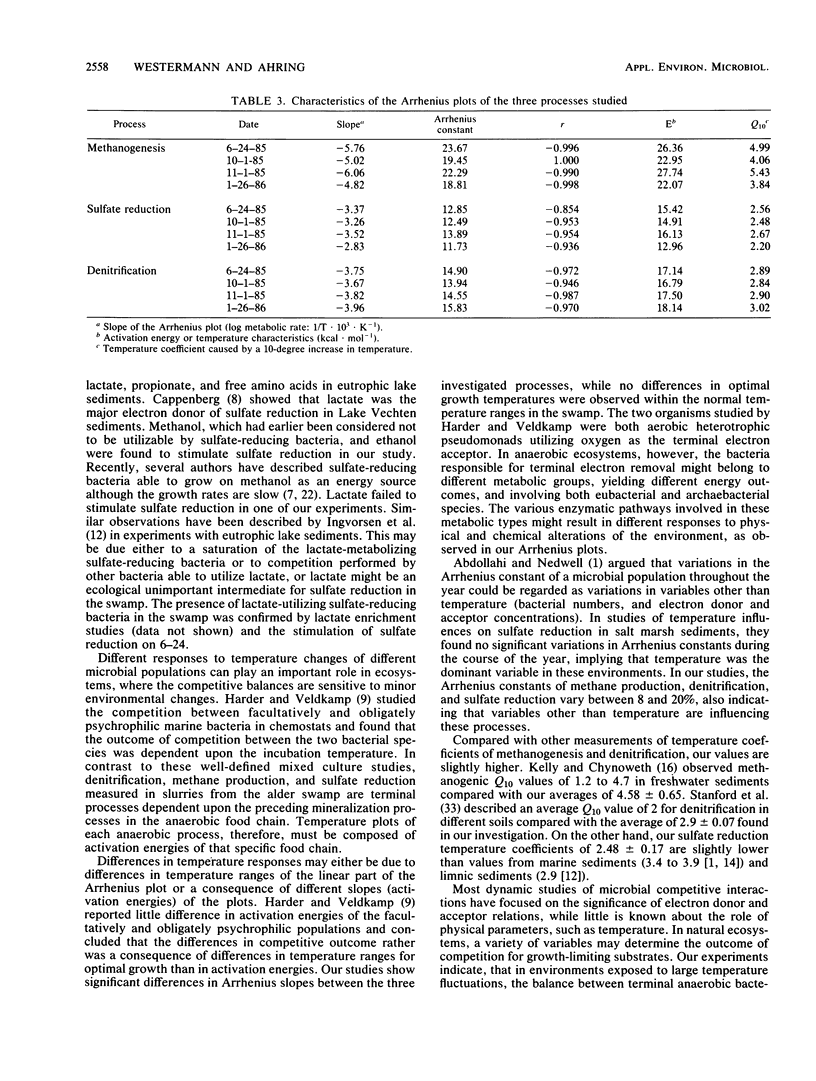
The dynamics of sulfate reduction, methane production, and denitrification were investigated in a permanently waterlogged alder swamp. Molybdate, an inhibitor of sulfate reduction, stimulated methane production in soil slurries, thus suggesting competition for common substrates between sulfate-reducing and methane-producing bacteria. Acetate, hydrogen, and methanol were found to stimulate both sulfate reduction and methane production, while trimethylamine mainly stimulated methane production. Nitrate addition reduced both methane production and sulfate reduction, either as a consequence of competition or poisoning of the bacteria. Sulfate-reducing bacteria were only slightly limited by the availability of electron acceptors, while denitrifying bacteria were seriously limited by low nitrate concentrations. Arrhenius plots of the three processes revealed different responses to temperature changes in the slurries. Methane production was most sensitive to temperature changes, followed by denitrification and sulfate reduction. No significant differences between slope patterns were observed when comparing summer and winter measurements, indicating similar populations regarding temperature responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram J. W., Nedwell D. B. Inhibition of methanogenesis by sulphate reducing bacteria competing for transferred hydrogen. Arch Microbiol. 1978 Apr 27;117(1):89–92. doi: 10.1007/BF00689356. [DOI] [PubMed] [Google Scholar]
- Balderston W. L., Payne W. J. Inhibition of methanogenesis in salt marsh sediments and whole-cell suspensions of methanogenic bacteria by nitrogen oxides. Appl Environ Microbiol. 1976 Aug;32(2):264–269. doi: 10.1128/aem.32.2.264-269.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banat I. M., Lindström E. B., Nedwell D. B., Balba M. T. Evidence for coexistence of two distinct functional groups of sulfate-reducing bacteria in salt marsh sediment. Appl Environ Microbiol. 1981 Dec;42(6):985–992. doi: 10.1128/aem.42.6.985-992.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. II. Inhibition experiments. Antonie Van Leeuwenhoek. 1974;40(2):297–306. doi: 10.1007/BF00394388. [DOI] [PubMed] [Google Scholar]
- Harder W., Veldkamp H. Competition of marine psychrophilic bacteria at low temperatures. Antonie Van Leeuwenhoek. 1971;37(1):51–63. doi: 10.1007/BF02218466. [DOI] [PubMed] [Google Scholar]
- Ingvorsen K., Zeikus J. G., Brock T. D. Dynamics of bacterial sulfate reduction in a eutrophic lake. Appl Environ Microbiol. 1981 Dec;42(6):1029–1036. doi: 10.1128/aem.42.6.1029-1036.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen N., Blackburn T. H. Seasonal rates of methane oxidation in anoxic marine sediments. Appl Environ Microbiol. 1981 Jun;41(6):1295–1300. doi: 10.1128/aem.41.6.1295-1300.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. Denitrification, acetylene reduction, and methane metabolism in lake sediment exposed to acetylene. Appl Environ Microbiol. 1979 Sep;38(3):486–493. doi: 10.1128/aem.38.3.486-493.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Dwyer D. F., Klug M. J. Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl Environ Microbiol. 1982 Jun;43(6):1373–1379. doi: 10.1128/aem.43.6.1373-1379.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga H. J., Gottschal J. C. Properties of Desulfovibrio carbinolicus sp. nov. and Other Sulfate-Reducing Bacteria Isolated from an Anaerobic-Purification Plant. Appl Environ Microbiol. 1987 Apr;53(4):802–809. doi: 10.1128/aem.53.4.802-809.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Umberger C., Culbertson C. W., Smith R. L. Denitrification in san francisco bay intertidal sediments. Appl Environ Microbiol. 1984 May;47(5):1106–1112. doi: 10.1128/aem.47.5.1106-1112.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A., Blackburn T. H. Estimation of sediment denitrification rates at in situ nitrate concentrations. Appl Environ Microbiol. 1979 Jan;37(1):174–176. doi: 10.1128/aem.37.1.174-176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior E., Lindström E. B., Banat I. M., Nedwell D. B. Sulfate reduction and methanogenesis in the sediment of a saltmarsh on the East coast of the United kingdom. Appl Environ Microbiol. 1982 May;43(5):987–996. doi: 10.1128/aem.43.5.987-996.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethunathan N., Rao V. R., Adhya T. K., Raghu K. Microbiology of rice soils. Crit Rev Microbiol. 1983;10(2):125–172. doi: 10.3109/10408418209113561. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Electron donors utilized by sulfate-reducing bacteria in eutrophic lake sediments. Appl Environ Microbiol. 1981 Jul;42(1):116–121. doi: 10.1128/aem.42.1.116-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Reduction of sulfur compounds in the sediments of a eutrophic lake basin. Appl Environ Microbiol. 1981 May;41(5):1230–1237. doi: 10.1128/aem.41.5.1230-1237.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol. 1982 Feb;43(2):319–324. doi: 10.1128/aem.43.2.319-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. T., Crawford R. L. Methane production in Minnesota peatlands. Appl Environ Microbiol. 1984 Jun;47(6):1266–1271. doi: 10.1128/aem.47.6.1266-1271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Brock T. D. Methane formation and methane oxidation by methanogenic bacteria. J Bacteriol. 1979 Jan;137(1):420–432. doi: 10.1128/jb.137.1.420-432.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Huser B., Brock T. D. Measuring radioactive methane with the liquid scintillation counter. Appl Environ Microbiol. 1979 May;37(5):897–899. doi: 10.1128/aem.37.5.897-899.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



