Abstract
Survival rates of dopamine (DA) neurons grafted to the denervated striatum are extremely poor (5-20%). Gene transfer of survival promoting factors, such as the anti-apoptotic protein bcl-2, to mesencephalic DA neurons prior to transplantation (ex vivo transduction) offers a novel approach to increase graft survival. However, specific criteria to assess the efficacy of various vectors must be adhered to in order to reasonably predict successful gene transfer with appropriate timing and levels of protein expression. Cell culture results utilizing three different herpes simplex virus (HSV) vectors to deliver the reporter ß-galactosidase gene (lacZ) indicate that transduction of mesencephalic cells with a helper virus-free HSV amplicon (HF HSVTH9lac) that harbors the 9-kb tyrosine hydroxylase (TH) promoter to drive lacZ gene expression elicits the transduction of the highest percentage (≈50%) of TH-immunoreactive (THir) neurons without significant cytotoxic effects. This transduction efficiency and limited cytotoxicity was superior to that observed following transduction with helper virus-containing HSV (HC HSVlac) and helper virus-free HSV amplicons (HF HSVlac) expressing lacZ under the transcriptional control of the HSV immediate-early 4/5 gene promoter. Subsequently, we assessed the ability of HSV-TH9lac and the bcl-2 expressing HSV-TH9bcl-2 amplicon to transduce mesencephalic reaggregates. Although an increase in bcl-2 and ß-galactosidase protein was induced by transduction, amplicon-mediated overexpression of bcl-2 did not lead to an increase in grafted THir neuron number. Even with highly efficient viral vector-mediated transduction, our results demonstrate that ex vivo gene transfer of bcl-2 to mesencephalic reaggregates is ineffective in increasing grafted DA neuron survival.
Keywords: Gene Transfer, Bcl-2, Herpes Simplex Virus, Mesencephalon, Parkinson's Disease, Ex vivo Transduction
2. Introduction
Parkinson's disease (PD) is a chronic, neurodegenerative disorder that affects approximately 1-2% of the population over the age of 65 [29]. The disease is caused by the specific degeneration of the dopaminergic neurons of the substantia nigra pars compacta, which is likely due to cumulative effects of genetic and environmental factors. Replacement strategies for PD, such as transplantation of primary embryonic dopamine (DA) neurons, are directed at restoring lost DA neurochemistry, returning brain function to the state that existed prior to the onset of symptoms of PD. Many years of successful research on neural grafting in animal models of PD [9, 50] have led to several clinical trials worldwide [31, 35].
While the field of transplantation continues to advance at a great pace, several challenges remain. In contrast to what might have been anticipated, double-blind clinical trials with grafting of DA neurons failed to provide clinical benefits for all patient groups and yielded dyskinetic behaviors [17, 30]. It was originally hypothesized that postoperative exacerbation of dyskinesias was due to graft overgrowth in the striatum and a generalized hyperdopaminergic effect. However, data from both clinical trials [26] and animal studies [28, 44] suggest that non-homogeneous DA fiber reinnervation is more likely causative. The percentage of grafted fetal DA neurons that survive transplantation is extremely low (5-20%), further limiting the ability of these grafted cells to provide innervation. The reported negative impact of grafting on dyskinesias does not imply that neural grafting has failed to fulfill its clinical promise, but rather that it remains critical to the field of cell replacement, whether the source is embryonic, stem or other, to understand means by which enhanced survival, and therefore enhanced and homogeneous DA fiber reinnervation, can be attained.
Gene therapy for the treatment of PD has mainly been directed at the delivery of trophic factors and dopaminergic enzymes to cells of the striatum (in vivo gene therapy) [42] or to nonneuronal cells that are subsequently grafted (ex vivo) [3, 16, 18, 22, 25, 47, 49]. Very little research has investigated the feasibility of ex vivo gene delivery to mesencephalic DA neurons in an effort to increase their survival rate after grafting. In our laboratory we have observed a peak of apoptotic nuclear profiles in mesencephalic grafts immediately after implantation (1-4 days) [38, 41]. These studies, along with previous findings [4, 13, 14], underscore the fact that the critical interval during which grafted DA neurons are dying in grafts to rats is during the first four days following implantation. Therefore, neuroprotection of DA neurons to be implanted will most effectively be accomplished via ex vivo transduction that generates optimal protein expression at the time of implantation.
In the present study we examine the ex vivo transduction of primary mesencephalic DA neurons and reaggregate cultures with different herpes simplex viral (HSV) vectors to derive an optimal set of transduction conditions and to assess the effects of amplicon-mediated delivery of the gene encoding the anti-apoptotic factor bcl-2 on graft survival and efficacy in vivo.
3. Results
Cytotoxicity of Vectors
Three HSV amplicon vectors were assessed for cytotoxic effects on transduced mesencephalic cultures in general (lactate dehydrogenase, LDH, assay) and on THir neurons specifically (counts of THir neurons). On DIV 4, all vectors displayed significantly higher LDH levels at an MOI of 2.0 when compared to their respective LDH values at MOI levels of 0.5 and 1.0 [F (8, 36) = 114.525; P ≤ 0.0001]. Across vectors, both the HC HSVlac and the HF HSVlac amplicons displayed significantly higher LDH levels than HF HSVTH9lac-transduced cultures at all three MOI levels examined (P ≤ 0.0003). These results are depicted in Figure 1A.
Figure 1. Cytotoxicity of Three Different HSV Amplicon Vectors.
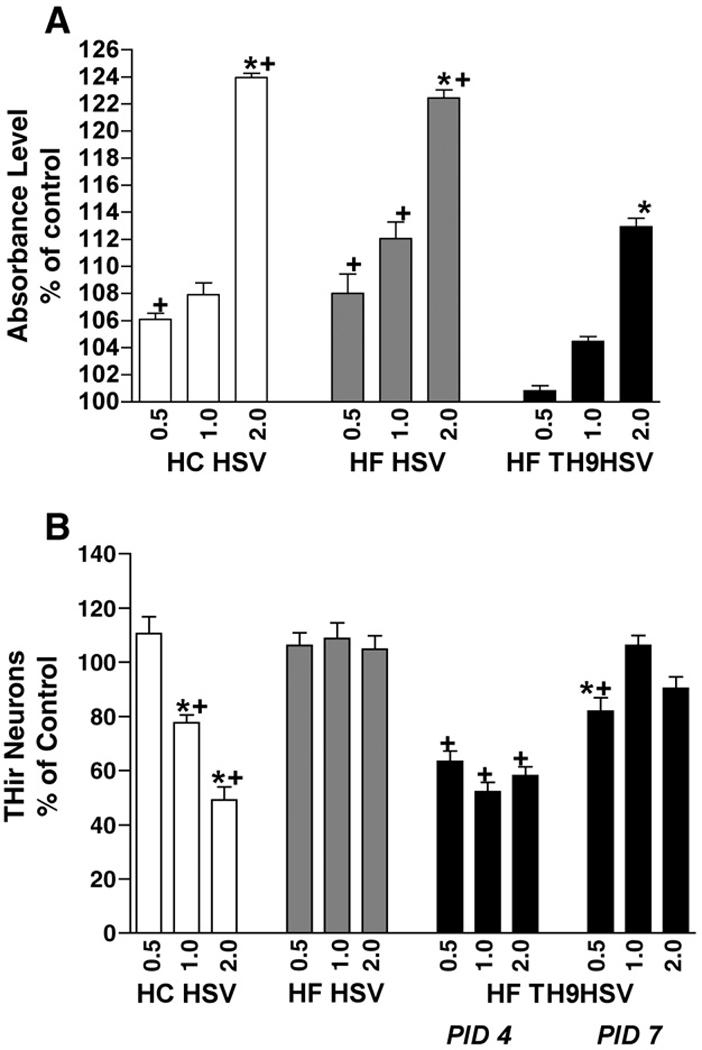
HC HSVlac, HF HSVlac and HF HSV-TH9lac were assessed for cytotoxic effects on transduced mesencephalic monolayer cultures. A. Lactate Dehydrogenase (LDH) Assay absorbance level results indicated that the highest multiplicity of infection level (MOI) of 2.0 all three vectors displayed significantly higher LDH levels than lower levels of the same vector (* = P < 0.0001, comparisons within vector treatments). Additionally, both HC HSVlac and HF HSVlac amplicons displayed significantly higher LDH levels than HF TH9lac (+ = P < 0.003, comparisons between same MOI, different vector treatments). B. Counts of THir neurons revealed that only HC HSV transduction yielded significantly fewer THir neurons due to higher levels of vector exposure (* = P < 0.0001, comparisons within vector treatments). No significant differences were observed in the number of THir neurons within cultures transduced at MOI levels of 1.0 and 2.0 with HF HSVlac on post infection day 4 (PID4) and HF TH9lac on PID7 (P ≥ 0.5, + = P < 0.0001, comparisons between same MOI, different vector treatments). Values represent the mean ± S.E.M.
Only HC HSVlac transduction yielded a dose-dependent decrease in THir neurons observed in culture at PID 4 [F (11, 49) = 27.342; P ≤ 0.0001]. There were no significant differences in THir neuron number due to increasing MOI levels of either HF virus examined (HF HSVlac or HF HSV TH9lac, P ≥ 0.5). Across vectors, infection with HF HSV TH9lac yielded significantly fewer THir neurons in culture than HF HSVlac when examined on post infection day 4 (PID4, P ≤ 0.0001). However, there were no significant differences in THir neuron number between HF HSVlac transduced cells at PID 4 and HF HSV TH9lac at PID 7 for MOI levels of 1.0 and 2.0 (P ≥ 0.5). These results are depicted in Figure 1B.
General Transduction Efficiency
Individual mesencephalic cultures transduced with the three amplicon constructs were examined on PID 4 and PID 7 for total number of β-gal positive cells (Figure 2, A and B). Cells infected at the time of plating with either HC HSVlac or HF HSVlac exhibited numerous transduced cells at the PID 4 and PID 7, while cultures incubated with HF HSV TH9lac at the time of plating were completely devoid of transduced cells at either timepoint (data not shown). Therefore, subsequent culture studies utilizing HF HSV TH9lac were transduced 24 h after plating.
Figure 2. Transduction Efficiency of Three Different HSV Amplicon Vectors.
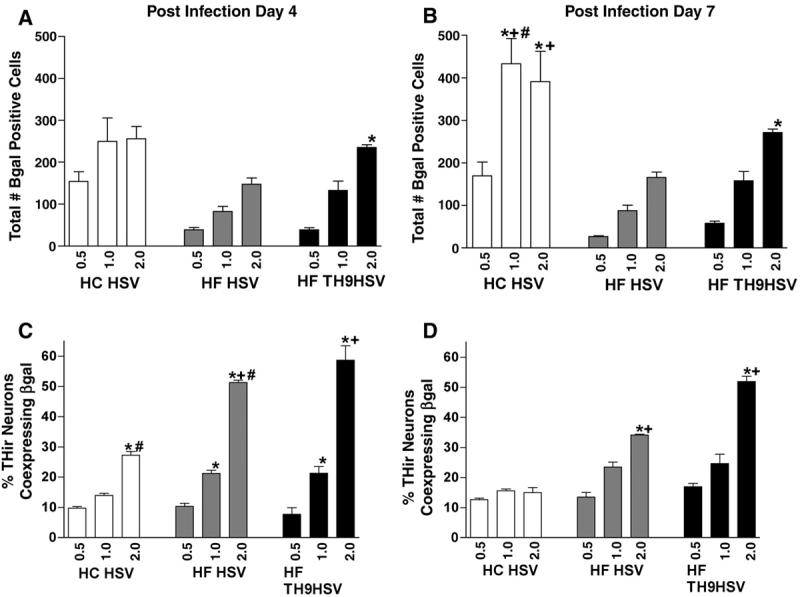
HC HSVlac, HF HSVlac and HF HSV-TH9lac were assessed for their ability to transduce mesencephalic cells in general (A, B) and THir neurons in particular (C, D). Analysis was conducted at either 4 days (A, C) or 7 days (B, D) after infection. HC HSV displayed significantly higher overall transduction efficiency at MOI concentrations of 1.0 and 2.0 on post infection day 7 (B, P < 0.0001) however 7 days after infection exposure to HF TH9-lac at an MOI of 2.0 generated significantly greater transduction efficiency of dopamine neurons within the cultures (D, P < 0.0001). * = P < 0.0001, comparisons within vector treatments; + = P < 0.003, comparisons between same MOI, different vector treatments. Values represent the mean ± S.E.M.
Within vector treatment groups, at PID 4, only HF HSV-TH9lac transduced cells displayed significantly higher numbers of β-gal positive cells when infected at an MOI of 2.0, compared to MOI 0.5 [F (17, 36) = 15.078; P ≤ 0.0001]. Neither HC HSVlac nor HF HSVlac transduced cells displayed significant differences in β-gal positive cell number with increasing MOI levels when examined at PID 4 (Figure 2A, P ≥ 0.5). Across vector treatment groups there were no significant differences in the number of β-gal positive cells observed at PID 4 (P ≥ 0.5).
Three days later (Figure 2B), at PID 7, HC HSVlac transduced cells at MOI levels of 1.0 and 2.0 displayed significantly higher numbers of β-gal positive cells compared to MOI level of 0.5 (P ≤ 0.0001). There were no significant differences in β-gal positive cell numbers between HC HSVlac transduced cells infected at MOI 1.0 and 2.0 (P ≥ 0.5). Similarly, at PID 7 mesencephalic cells infected with HF TH9HSVlac at a MOI level of 2.0 displayed significantly more β-gal positive cells than those infected at MOI 0.5 (P ≤ 0.0001). There were no significant differences with increasing MOI levels of HF HSVlac transduced cells in β-gal positive cell number (P ≥ 0.5). Across vector constructs, at PID 7, at an MOI of 1.0, HC HSVlac infected cells displayed significantly more β-gal positive cells than HF HSVlac and HF HSV-TH9lac infected cells at the same MOI (P ≤ 0.0001). Similarly, cells infected with HC HSVlac at MOI of 2.0 displayed significantly more β-gal positive cells than cells infected with HF HSVlac (P ≤ 0.0001). There were no significant differences in β-gal positive cell number between HC HSVlac infected and HF HSV-TH9lac infected cells at an MOI of 2.0 (P ≤ 0.0001). Across time, only HC HSVlac infected cells displayed a significant increase in β-gal positive cells at PID 7 as compared to PID 4, and only when infected at a MOI of 1.0 (P = 0.0001). No other significant effects due to post transduction time were observed (P ≥ 0.5).
THir Neuron Specific Transduction Efficiency
Individual mesencephalic cultures transduced with the three vector constructs were also examined on PID 4 and PID 7 for the percentage of THir neurons that co-expressed β-gal (Figure 2C and D). At PID 4, HC HSVlac infected cells displayed significantly more THir neurons (approximately 27%) transduced at a MOI of 2.0, compared to cells infected at lower MOIs (P ≤ 0.0001). HF HSVlac transduced cultures possessed more double-labeled neurons when infected at MOI 2.0 (≈ 52%) when compared to MOI 1.0 (P = 0.0002), and more double-labeled neurons at MOI 1.0 when compared to MOI 0.5 (P ≤ 0.0001). Similarly, HF HSV-TH9lac transduced THir neurons in a dose-dependent manner (≈57% at MOI 2.0, P ≤ 0.0001). Both helper free viral constructs transduced significantly more THir neurons at PID 4 than HC HSVlac when infected at MOI 2.0 (P ≤ 0.0001). There were no significant differences between treatment groups with any other statistical comparison (P ≥ 0.5).
At PID 7 (Figure 2D) there were no significant differences in transduced THir neuron number due to MOI level for HC HSVlac infected cells (P ≥ 0.5), all levels exhibited approximately 15% THir neurons co-expressing β-gal. In contrast, both helper virus-free amplicon stocks elicited significant increases in double-labeled neurons at MOI 2.0 as compared to lower MOIs (P ≤ 0.0002). At MOI 2.0, HF HSVlac transduced THir neurons at a significantly higher rate than HC HSVlac at the same MOI (≈ 35% and ≈15%, respectively, P ≤ 0.0001). However, mesencephalic cultures transduced with HF HSV-TH9lac at MOI 2.0 possessed the highest percentage of double-labeled neurons at PID 7, approximately 50%. This rate was significantly higher than then other two vectors at the same MOI (P ≤ 0.0001). Across time, both HC HSVlac and HF HSVlac transduced cultures displayed significantly fewer β-gal positive THir neurons at PID 7 than at PID 4 when infected at MOI 2.0 (P ≤ 0.0001). In contrast, there was no significant difference in transduction rates of THir neurons in HF HSV-TH9lac transduced cultures at PID 4 and (P ≥ 0.5).
Transduction of Mesencephalic Reaggregates
Mesencephalic reaggregates were analyzed for expression of ß-galactosidase or bcl-2 protein at PID 3 or 5 after exposure to either HF HSV-TH9lac, HF HSV-TH9bcl-2 or no vector utilizing immunohistochemistry, X-gal histochemistry and Western blotting. Qualitative examination of sections taken through the center of the aggregates revealed numerous X-gal positive cells at PID 4 after infection with HF TH9HSVlac (Figure 3A). Similarly, at PID 4, aggregates transduced with HF HSV-TH9bcl-2 displayed numerous bcl-2 positive cells (Figure 3B). Alternate sections through the aggregates transduced with HSV-TH9lac indicated numerous THir neurons (Figure 3C). At both PID 4 and PID 7 reaggregates transduced with HF HSV-TH9lac displayed higher levels of ß-galactosidase than reaggregates infected with HF HSV-TH9bcl-2 or no vector. Similarly, transduction of reaggregates with HF HSV-TH9bcl-2 yielded greater expression of bcl-2 protein at both PID 4 and PID 7 than reaggregates transduced with non-bcl-2 expressing amplicons (data not shown).
Figure 3. Transduction of Mesencephalic Reaggregates.
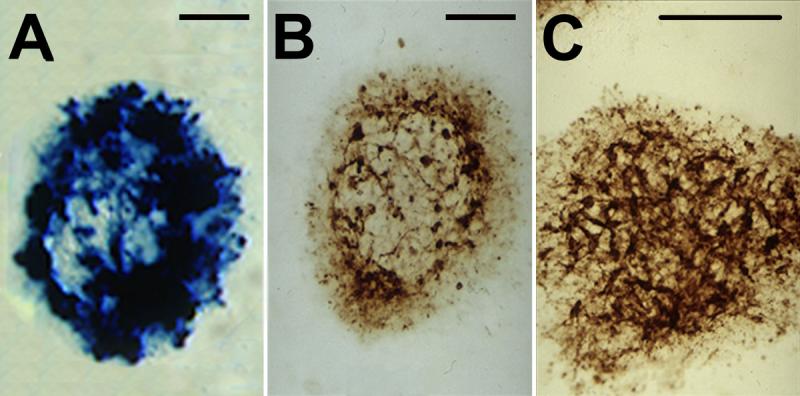
Cross sections were taken through mesencephalic reaggregates 4 days after infection with either HF HSV-TH9lac (A, C) or HF HSV-TH9bcl-2 amplicon vectors. X-gal histochemistry and bcl-2 immunohistochemistry (A and B respectively) reveal efficient transduction of mesencephalic reaggregates. These same reagreggates possess numerous THir neurons (C). Scale bars in A, B, C = 100 μm.
Rotational Asymmetry
Rats in all transplant groups were assessed for the effects of freshly dissociated and reaggregate grafts (transduced and control) on rotational asymmetry. Thirty-two of the forty 6-OHDA-lesioned rats exhibited average ipsilateral rotation rates of 6 turns/minute or greater after amphetamine challenge and were assigned to the four different treatment groups. There were no significant differences in baseline rotational scores between the different transplant groups [F (11,67) = 1.941; P ≥ 0.05]. At four weeks following grafting, the group that had been grafted with freshly dissociated mesencephalic suspension (Susp, N = 8) appeared to have recovered slightly from baseline, however this did not achieve significance (P ≥ 0.05). Similarly, there was no significant recovery from rotational asymmetry in any of the three reaggregate grafted groups (Control, N = 6; Lac, N = 7; Bcl-2, N = 6; P ≥ 0.05). In contrast, by six weeks after transplantation all four groups displayed average ipsilateral rotation rates that were significantly recovered compared to baseline (P ≤ 0.05). There were no significant differences in rotational asymmetry between groups (P ≥ 0.05). Rats with misplaced transplants (lateral ventricle) were excluded from behavioral analysis. These behavioral results are illustrated in Figure 4A.
Figure 4. Effects of Bcl-2 Transduction on Graft-Induced Recovery from Rotational Asymmetry and Grafted THir Neuron Survival.
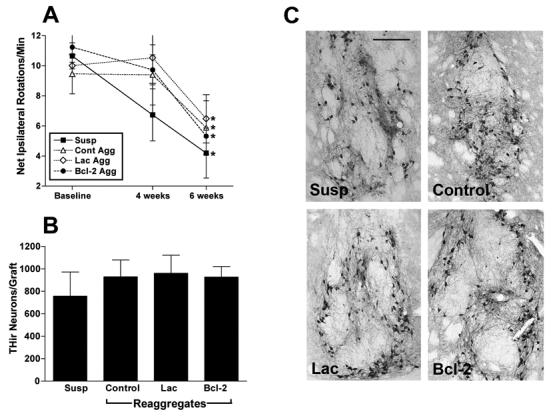
A. Unilaterally lesioned rats received grafts of equivalent numbers of mesencephalic cells that were either freshly put into suspension (Susp), aggregated in rotary culture for 3 days (Cont Agg), aggregated and transduced with HF HS TH9lac (Lac Agg) or aggregated and transduced with HF HSV TH9bcl-2 (Bcl-2 Agg). Six weeks after transplantation all graft treatments displayed statistically similar behavioral recovery profiles (* = P < 0.05, compared to baseline rotational scores). B. No significant difference was observed between treatment groups in survival of grafted THir neurons 10 weeks after implantation. Values represent the mean ± S.E.M. C. Representative photomicrographs of THir neurons within grafts of mesencephalic cell suspension (Susp), control mesencephalic reaggregates (Control), aggregates transduced with HF HSV TH9lac (Lac) and aggregrates transduced with HF HSV TH9bcl-2 (Bcl-2). Scale bar in A = 250 μm.
Survival of THir Neurons
Unilateral injection of 6-OHDA produced a near total absence of TH immunoreactivity within the lesioned host striatum. All grafted rats in both experiments contained visible grafts of THir neurons when analyzed at 10 weeks following implantation. Experimental groups consisted of freshly suspended VM grafts (Susp, N = 8), non-transduced reaggregate grafts (Control, N = 8), reaggregate grafts transduced with HSV TH9lac (Lac, N = 8), and reaggregate grafts transduced with HSV TH9bcl-2 (Bcl-2, N = 7, one rat died due to vivarium complications). Fresh VM suspension grafts possessed an average of 757.05 ± 215.5 THir neurons (survival rate approximately 4.2%) when analyzed at the ten-week post-implantation interval. Non-transduced reaggregate grafts displayed an average of 929.88 ± 149.87 THir neurons (survival rate approximately 4.9%). Reaggregate grafts transduced with HSV-TH9lac displayed an average of 961.7 ± 160.6 (survival rate approximately 5.0%) THir neurons. Lastly, reaggregate grafts transduced with HSV-TH9bcl2 displayed an average of 927.7 ± 93.9 (survival rate approximately 4.9%) THir neurons. There were no significant differences in survival rates of THir neurons between the four different graft types (P ≥ 0.05). These results are depicted in Figure 4B-C.
Coexpression of β-galactosidase and TH in HSV-TH9lac Transduced Grafts
In order to verify the transduction of THir neurons after transplantation, rats grafted with HF HSV-TH9lac were analyzed for coexpression of TH and β-gal (Figure 5). HSV-TH9lac transduced grafts contained an average of 22.0 ± 4.3 double labeled neurons (approximately 2.2% of THir neurons). Interestingly, these same grafts contained an average of 34.0 ± 7.9 total β-gal positive cells, indicating that not all β-gal positive cells expressed TH (approximately 65% coexpression).
Figure 5. Coexpression of TH and β-gal in Mesencephalic Grafts.
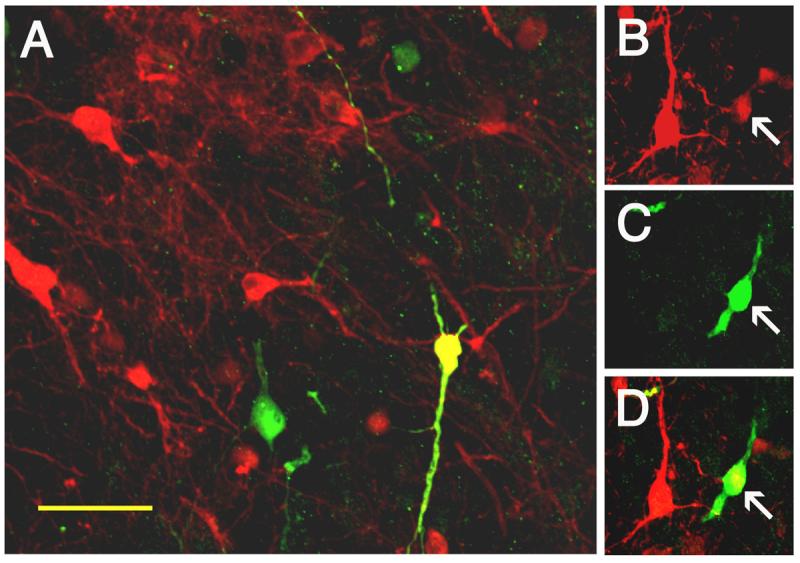
Mesencephalic reaggregates were transduced with HF HSV-TH9lac, implanted to the denervated striatum of rats and analyzed using double label immunofluorescence. A. 10 weeks after transplantation β-gal-ir cells (green), THir neurons (red) and neurons coexpressing both β-gal and TH (yellow) are observed. B. An individual grafted neuron (arrow) expressing TH, B. Expressing β-gal and C. Merged images. Scale bar in A = 15 μm.
4. Discussion
The present group of experiments systematically evaluates three different HSV vector constructs for their ability to efficiently transduce embryonic mesencephalic DA neurons without eliciting cytotoxicity. We demonstrate that a helper virus-free preparation of a HSV vector that uses the 9-kb TH promoter to drive expression is optimal for the purpose of transducing THir neurons within the heterogeneous mesencephalic monolayer culture population. We provide evidence to validate that three dimensional mesencephalic reaggregates similarly are efficiently transduced within 3 days of HF HSV-TH9 vector exposure to express either ß-galactosidase or bcl-2 protein as confirmed by both immunohistochemistry and Western blots. With optimal transduction protocols identified, combined with confirmation of protein expression at the time of grafting, we were appropriately poised to definitively determine whether bcl-2 overexpression is capable of increasing grafted DA neuron survival. Our results indicate that gene transfer of bcl-2 does not prevent the cell death experienced by grafted cells after implantation and therefore does not represent an effective approach to increase grafted DA neuron survival.
The assessment of the potential toxicity of overexpression of native or exogenous proteins is critical to successful gene therapy. Previous studies in which primary cortical neurons were transduced with either helper or helper-free HSV packaged amplicon virus stocks expressing different reporter genes indicated that the toxicity observed in these cultures was related to the type of reporter gene expressed, not the presence of helper virus, with lacZ expression the least toxic of the reporter genes examined [12]. In our studies using primary mesencephalic cells we found similar results in that the presence of helper virus did not impact the toxicity observed in the mesencephalic cultures in general. However, the presence of helper virus was significantly more toxic to the THir neurons in these same cultures.
Transduction of mesencephalic cultures with the HF TH9lac vector construct yielded significantly lower THir neurons at PID 4 compared to cultures transduced with HF HSVlac. However, by PID 7 the number of THir neurons in cultures transduced with TH9lac (MOIs 1.0, 2.0) had returned to the numbers observed with HF HSVlac transduction. This transient loss of TH phenotype is not indicative of toxicity of the TH9lac vector. In fact, analysis of LDH levels reveal that the TH9lac vector construct is significantly less toxic than both the HC and HF HSV vectors. Based on these results, as well as our results demonstrating superior transduction rate of THir neurons, we selected the HF TH9lac vector construct for our transplantation studies.
We have previously reported that apoptotic cell death of grafted cells peaks immediately after implantation [38, 41]. This underscores the concept that the fate of grafted DA neurons is determined during the immediate post-grafting interval, with survival after 4 days being equivalent to survival rates observed months later [4, 13, 14, 38, 41]. Therefore, interventions aimed at augmenting grafted dopamine survival must target these critical first few days after implantation. Our in vitro results utilizing both monolayer cultures and aggregates suggests significant expression of the bcl-2 within THir neurons at the time of implantation (≈ 50%). However, by ten weeks after implantation the transduction rate of THir neurons had decreased markedly to approximately 2%. This down-regulation of transgene expression after only a few days is a prevailing characteristic of HSV amplicon-based vectors [12, 46] although slightly longer expression stability has been reported by using the TH promoter [48]. For the purposes of improving graft survival, down-regulation of bcl-2 after a few days should prove adequate and in fact, may be optimal. With HSV-TH9bcl-2-mediated transduction bcl-2 expression is in essence “regulatable” with bcl-2 expression maximal when newly grafted cells are bombarded by numerous triggers of cell death [37] and subsequently down-regulated when these challenges have past.
Bcl-2 is a member of a gene family that can be functionally subdivided into two groups with one group promoting apoptotic cell death (Bax, Bak) and the other group suppressing it (Bcl-2, Bcl-Xl) [51]. Our present findings rule out bcl-2 overexpression as a method to increase grafted DA neuron survival. However, bcl-2 overexpression does protect nigral DA neurons from MPP+ toxicity in culture [33] and programmed cell death during development [23]. Given these findings it appears that the ability of bcl-2 overexpression to provide neuroprotection for nigral DA neurons depends upon the type of insult. Newly grafted DA neurons experience very specific kinds of insults that are unique to the grafting situation, i.e. anoikis, hypoxia/ischemia, trophic factor withdrawal [37].
This study is not the first attempt to utilize bcl-2 overexpression to protect grafted DA neurons after transplantation. Initially, an immortalized cell line derived from rat mesencephalon was transduced with a retrovirus encoding bcl-2 and subsequently grafted [2]. Two different groups have also examined the effect on graft survival of grafting mesencephalic suspensions from mice genetically engineered to overexpress bcl-2 [1, 36]. None of these three studies reported differences in cell survival between the bcl-2 overexpressing treatment groups and controls; however, enhanced THir neurite extension with bcl-2 overexpression was reported [21, 36]. This finding is not surprising given that the bcl-2 molecule has been demonstrated to have the capacity to induce and maintain axonal growth [10]. The authors concluded from their findings that cell death in grafted cells can circumvent regulation by bcl-2 [36]. While our present findings support this conclusion, these studies could not rule out the possibility that inadequate levels of bcl-2 were expressed at the time of implantation to provide protection. Given that grafted cells die immediately following implantation [38, 41], bcl-2 must be overexpressed during this interval in order to promote graft survival. While the aforementioned studies confirmed bcl-2 overexpression, this confirmation was not conducted immediately prior to implantation. Instead, verification of bcl-2 protein expression was conducted via Western blots of mesencephalic tissue pieces, post mortem examination of transgenic tissue or in vitro at 48 hours after plating [21, 36]. Bcl-2 overexpression was not demonstrated after tissue dissociation, a disruptive process that can temporarily interrupt protein expression and render cells quiescent. It is possible that the stress of dissociation into cell suspension downregulated bcl-2 to physiologically inactive levels during the immediate post-grafting interval. We propose that our mesencephalic reaggregate system has eliminated this potential confound thereby allowing us to definitively determine that bcl-2 overexpression, at least at the present levels, does not elicit survival promoting effects.
One advantage of gene transfer to cells prior to implantation (ex vivo gene therapy) is the ability to methodically evaluate the extent and cell-specificity of transduction prior to implantation. In the case of primary mesencephalic cell suspensions, this opportunity is critical to predicting success. Mesencephalic cells are a heterogeneous mixture of neurons (GABAergic, serotonergic, dopaminergic) as well as glia. The THir cell population within this diverse cell mixture varies (dependent on dissection size) from around 1-20%. If the gene of interest to be used to promote DA neuron survival must be expressed by the DA neurons themselves in order to elicit survival effects then the ability of the vector to infect a significant percentage of THir neurons must be established. Our experimental design has allowed us to verify that THir neurons are indeed transduced with the HF HSVTH9 vector (≈50%). Conversely, if THir neurons do not require expression of the gene and protein to be investigated in order to elicit trophic effects then transduction of the nondopaminergic cell population may suffice. This latter situation may be most applicable to ex vivo transduction of mesencephalic cells with trophic factors such as glial cell line derived neurotrophic factor (GDNF) or brain derived neurotrophic factor (BDNF).
Significant agreement has emerged from the work of researchers worldwide that specific conditions associated with the transplant procedure render grafted cells susceptible to apoptotic death. The potential triggers of apoptosis during the transplantation procedure include: hypoxia, anoikis, oxidative stress and neurotrophic factor withdrawal. Treatment strategies that aim to reduce or eliminate the triggers of grafted cell death appear to be more successful than approaches that target the downstream intracellular apoptotic cascade [37]. In particular, treatment of mesencephalic cell suspensions with isolated trophic factors (GDNF, BDNF, NT 4/5) as well as glial-derived factors, and antioxidant therapies have demonstrated consistent survival promoting effects. Nevertheless, despite significant improvements in grafted DA neuron survival using these treatments, the overwhelming majority of grafted embryonic cells (60-70%) still do not survive the grafting procedure. Perhaps the application of gene therapy delivery of trophic factors via carefully optimized ex vivo transduction could further improve upon the survival rates observed with trophic factor protein incubation. Future studies are underway to investigate the effectiveness of such an approach.
5. Experimental Procedures
Dissection and Dissociation
Ventral mesencephalic brain regions were dissected using sterile techniques from embryonic day 14 F344 rat fetuses and pooled in a cold, sterile calcium-magnesium-free buffer (CMF) as described previously [38, 39, 40, 41]. Cell suspensions of embryonic mesencephalic tissue were then prepared through a series of CMF rinses, incubated in 0.125% trypsin for ten minutes at 37°C, rinsed in CMF again, and triturated in 0.004% DNase to disperse the cells into solution. Trypan blue was added to a sample of cell suspension and viewed in a hemocytometer to assess cell viability and to determine cell counts. Each mesencephalon yielded approximately 600,000 cells under our dissection parameters, an average of 40 embryos was routinely dissected from 3-4 dams. Cell suspensions of greater than 95% viability were used for cell culture, aggregation and transplantation.
Preparation of amplicon vectors: Cell culture
Baby hamster kidney (BHK) and RR1 cell lines were maintained as previously described [24]. The NIH-3T3 mouse fibroblast cell line was originally obtained from American Type Culture Collection and maintained in Dulbecco's modified Eagle medium plus 10% fetal bovine serum.
Amplicon Construction
The coding sequence for E. coli ß-galactosidase was cloned into an HSV amplicon plasmid vector as previously described [19]. An HSV amplicon expressing bcl-2 under the control of the TH promoter was constructed. To obtain pHSVTH9bcl-2, a 750-bp cDNA fragment from human bcl-2 was used (a gift from Dr. Arnon Rosenthal). This fragment was ligated into an HSV amplicon vector containing the rat tyrosine hydroxylase promoter. Specifically, a SalI-EcoRI fragment from pTH900 (a kind gift of Dr. Jin Son) containing 9 kb of the rat tyrosine hydroxylase promoter was subcloned into the Xho I-Eco RI sites of the HSVminOriSmc amplicon vector to create pHSV-TH9. pHSV-TH9 was subsequently linearized with Eco RI and ligated to the 750 bp Eco RI fragment of human bcl2 cDNA. The final construct, pHSV-TH9bcl2 is capable of TH promoter-directed expression of bcl-2.
Helper virus-based amplicon packaging (HC HSV)
Amplicon DNA was packaged into HSV-1 particles using a previously published method [32]. Viral pellets were resuspended in PBS (Ca2+ and Mg2+ free) and stored at −80°C for future use.
Helper virus-free amplicon packaging (HF HSV)
Amplicon stocks were also prepared using a modified helper virus-free packaging method [6]. Viral preparations were subsequently purified and concentrated as performed for helper virus-containing stocks.
Virus Titering
Helper virus-containing stocks (HC HSV) were titered for helper virus by standard plaque assay methods [20]. Amplicon titers for both helper virus-based and helper-free stocks (HF HSV) were determined using a previously published transduction titering method [5]. Helper virus titers (pfu/ml), amplicon expression titers (bfu/ml), and amplicon transduction titers (TU/ml) obtained from these methods were used to calculate amplicon titer and thus standardize experimental viral delivery. Amplicon titers of the various virus preparations ranged from 1-2 × 107 bfu/ml while helper titers were in the range of 0.5-1 × 107 pfu/ml.
Transduction of Mesencephalic Cells in Monolayer Cultures
All cell culture experiments were performed in triplicate. Primary dispersed mesencephalic cultures were plated at a concentration of 3000 cells/μl using the microisland dry plating method [45] to allow for standardized determinations of THir cell survival and transduction efficiency. Microislands 10 μl in size were plated on 24-well poly-D-lysine and primaria-coated culture plates and fed after one hour with serum-free Neurobasal media including 1X B-27 supplement, 25 μM L-glutamic acid, 2.0 mM glutamine, 10 U/ml penicillin, and 2.5 μg/ml fungizone in order to support mesencephalic neurons in a relatively glial-free condition [7, 8]. After feeding, cells were exposed immediately to varying multiplicity of infection levels (MOIs 0.5, 1.0, 2.0) of HC HSVlac, HF HSVlac or HF HSV-TH9lac. Initial results with HF HSV-TH9lac revealed no transduction of any cells when virus was added at the time of plating. Therefore, all cell culture experiments with HSV-TH9 amplicons were subsequently conducted with exposure to vector at DIV 1. Twenty-four hours after transduction, the medium was changed. Cultures were analyzed 4 (post infection day 4, PID 4) or 7 days (PID 7) later for number of THir neurons, β-galactosidase immunoreactive (βgal-ir) cells, and cells that colocalized for both antigens using confocal microscopy (see methods below). For the lactate dehydrogenase (LDH) assay, mesencephalic cells were plated 200,000 cells/well in 96-well poly-D-lysine and primaria-coated culture plates in 200 μl Neurobasal medium, transduced with vectors as described above, medium changed at 24 hs and subsequently analyzed at PID 4.
Lactate Dehydrogenase (LDH) Assay
Transduced cultures were analyzed for rates of necrotic cell death by assaying LDH release to the media. LDH release to the media is a measure of membrane integrity. On PID 4, medium samples from mesencephalic cells in 96-well plates and medium blanks were incubated for 30 min. at a 2:1 ratio with equal parts substrate, enzyme and dye solutions from the LDH Based In Vitro Toxicology Assay Kit (Sigma, St. Louis, MO). Next, 1 N HCl was added to stop the reaction and absorbance measured immediately at a wavelength of 490 nm on a spectrophotometer. Background medium measurements were subtracted to determine appropriate totals for each condition. This experiment was conducted three times.
Mesencephalic Reaggregates
Mesencephalic reagggregates were generated as described previously [39, 40]. 3 × 106 mesencephalic cells were added to sterile 10 ml Erlenmeyer flasks containing 2.5 ml of 50% striatal O-2A conditioned medium [40] with 10% fetal bovine serum (FBS). Mesencephalic cell-containing flasks were gassed for 10 min. each with a blood gas mixture, stoppered and placed in a rotary incubator at 90 rpm and 37°C. After twenty-four hours small reaggregates formed and maintained for a total of 3-5 days. Media was changed on day 2 and flasks were gassed with blood gas mixture every other day.
Transduction of Mesencephalic Reaggregates
Twenty-four hours after aggregation, aggregates for transduction with HF HSV-TH9bcl2 or HF HSV-TH9lac were exposed to the vector at a MOI of 2.0. Non-infected aggregates were also cultured as a negative control condition. Aggregates were rinsed 24 h later, regassed and re-fed with fresh 50% striatal O-2A conditioned medium containing 10% FBS. Reaggregates were assayed on PID 4 and PID 7 for protein expression via Western blot.
Western Blotting
Western blots were conducted according to previously described methods [12]. In brief, control and transduced mesencephalic reaggregates were harvested in ice-cold phosphate-buffered saline (pH = 7.2) containing protease inhibitors (1 mg/ml each of leupeptin, aprotinin and pepstatin A), and protein preparations were denatured in sodium dodecyl sulfate (SDS) loading buffer. Sample proteins (10 μg/sample) were separated by SDS polyacrylamide gel electrophoresis (20-25%, Longlife Microgel™, Life Therapeutics Ltd., Australia) and transferred to polyvinylidene fluoride membranes (Immobolin P, Millipore, Bedford, MA) electrophoretically. Blots were blocked in TBS/0.1% Tween-20/5% milk and incubated overnight at 4°C with mouse anti-bcl-2 (1 μg/ml, Research Diagnostics, Flanders, NJ), rabbit anti-β-galactosidase (1:5000; Cortex Biochem) and mouse anti-β-actin (1:10,000; Abcam, Cambridge, UK) antibodies in blocking buffer. After several rinses the blots were incubated for 1 hour at room temperature in either horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG secondary antibody (1:10,000; ICN, Costa Mesa, CA). Immunoreactive proteins were visualized by enhanced chemiluminescence (Kirkegaard & Perry, Gaithersburg, MD) and quantified using NIH Image 1.62. β-gal-ir and Bcl-2ir signals were normalized to β-actin signals on the same blot for quantitative analysis.
X-Gal (5-bromo-4-chloro-3-indoyl- β- D-galactoside) Histochemistry
For detection of ß-galactosidase, sections through gelatin embedded reaggregates were rinsed in 0.1M PO4 buffer and incubated overnight at 37° C in x-gal/iron/PBS solution consisting of 6.12 mM 5-bromo-4-chloro-3-indoyl- β- D-galactoside, 5 mM FeK3(CN)6, 5 mM K4Fe(CN)6, 0.2% NP-40, 0.01% sodium deoxycholate, and 2 mM MgCl2. Reacted sections were rinsed in 0.1M PO4 buffer followed by distilled H2O before coverslipping.
Unilateral Nigrostriatal Lesions
Stereotaxic injections of 6-hydroxydopamine (6-OHDA) were made unilaterally to the nigrostriatal pathway of anesthetized (30 mg/kg, pentobarbital) 40 male Fischer 344 rats (F344, 200-225 grams). Each rat received two injections of 6-OHDA at a concentration of 5.0 μg/μl at a rate of 1 μl/min for 2 min., one in the vicinity of the medial forebrain bundle (AP −4.3, ML +1.2, DV −7.5) and the other in the pars compacta of the substantia nigra (AP −4.8, ML +1.5, DV −7.5). Baseline measures of amphetamine-induced rotation were obtained at least 2 weeks following the 6-OHDA lesion of the nigrostriatal pathway. Lesioned animals were given an injection of 5.0 mg/kg (i.p.) amphetamine and placed in cylindrical bowls in which rotational behavior was observed and quantified by a Macintosh rotometer program 5 min. after injection for a period of 85 min. Animals meeting the criterion of 6 turns/minute (averaged over 85 minutes) or greater (n = 32) were randomly assigned to transplant groups and grafted 2 weeks later. Behavioral assessment was conducted at 4 and 6 weeks after grafting. Completeness of the lesion was verified histologically at the conclusion of the experiment.
Grafting Parameters
Rats were assigned to one of four different treatment groups; Group 1: Suspension Group (Susp) received grafts of freshly suspended embryonic mesencephalon on the day of dissection (to control for the effects of aggregation); Group 2: Control Reaggregates (Control) received grafts of mesencephalic reaggregates not exposed to viral vector; Group 3: ß-galactosidase Transduced Reaggregates (Lac) exposed to HF HSV-TH9lac; and Group 4: Bcl-2 Transduced Reaggregates (Bcl-2) exposed to HF HSV-TH9bcl2. All reaggregates were formed in rotary culture over the course of 3 days. Dissociated mesencephalic cells for the fresh control group (Group 1) were loaded into a 10 μl syringe with a 25-gauge needle and attached to a stereotaxic needle holder for implantation. Anesthetized rats (30 mg/kg, pentobarbital) were grafted via stereotaxic injections of 300,000 mesencephalic cells in 3 μl into the dorsomedial region of the striatum (AP +0.7, ML +2.5, DV −6.5). Based on previous estimates [15], this represents the implantation of approximately 18,500 THir cells per rat under our dissection parameters. For the fresh cell suspension transplant group, cells were diluted to 100,000 cells/μl in Hank's buffer solution (Gibco, Grand Island, NY) and grafted immediately (see methods below). The remaining cells were utilized for aggregation. After 24 h in the flasks, reaggregates had formed and were exposed to either HF HSV-TH9lac (Group 3) or HF HSV-TH9bcl2 (Group 4) at MOI 2.0, or no vector (Group 2) to provide an aggregate control condition. At day in vitro 3 (DIV 3), mesencephalic cells in reaggregate form were grafted to the remaining three groups of rats. The number of aggregates per flask was counted, and the appropriate number (1/10th) transplanted to the same above coordinates via a 25G spinal needle to represent 300,000 mesencephalic cells from the original cell suspension. All mesencephalic cells utilized within an experiment originated from the same E14 dissection to control for THir neuron number. Grafts found to be located outside of the dorsomedial region of the striatum were excluded from behavioral analysis.
Immunohistochemistry
For all cell culture immunohistochemistry, the media was removed and the cells rinsed in Tris Buffer (pH = 7.3), fixed in 4% paraformaldehyde in 0.1M PO4 buffer for 25 min. and rinsed in Tris Buffer again. For analysis of mesencephalic reaggregates, aggregates were rinsed in 0.9% NaCl followed by a 30-min. incubation in 4% paraformaldehyde and subsequent rinses in Tris buffer. For analysis of grafted tissue, 10 weeks after implantation rats were deeply anesthetized (60 mg/kg, pentobarbital, i.p.) and perfused intracardially with 4% paraformaldehyde. Brains were removed, postfixed for 24 hours in 4% paraformaldehyde and sunk in 30% sucrose in 0.1M PO4 buffer. Brains and gelatin-embedded reaggregates were frozen on dry ice and sectioned at a 35-μm thickness using a sliding microtome. Every sixth section through the graft was analyzed for surviving THir neurons; all sections through the aggregates were immunostained. Microtome sections were immunostained using the free-floating method. Following blocking in 10% normal goat serum, tissue sections and cells were incubated in primary antisera directed against TH (Chemicon, mouse anti TH 1:4,000), β-galactosidase (Cortex Biochem, rabbit anti β-gal, 1:500) or bcl-2 (DAKO, mouse anti BCL2 oncoprotein 1:200) overnight at room temperature. Triton-X (0.3 %) was added to the Tris buffer during incubations and rinses to permeabilize cell membranes. Following primary incubation, sections were incubated in biotinylated secondary antisera against mouse IgG (Chemicon, 1:400) followed by the Vector ABC detection kit employing horseradish peroxidase. Antibody labeling was visualized by exposure to 0.5 mg/ml 3,3' diaminobenzidine (DAB) and 0.03% H2O2 in Tris buffer. Sections were mounted on subbed slides, dehydrated to xylene and coverslipped in Pro-Texx. Manual counts of THir and βgal-ir cells within grafted tissue sections were made at 20X, the sum of these counts was then adjusted according to the method of Abercrombie [1]. Manual counts of immunocytochemically identified THir neurons in cell culture experiments were made in nine different center microisland fields at 20X as described previously [27, 40]
Immunofluorescence
Antisera for immunofluorescence included those directed against TH (Chemicon, mouse anti TH, 1:500) and β-galactosidase (Cortex Biochemicals, rabbit anti β-gal, 1:500). After fixation, blocking in 10% goat serum and permeabilization as described above, cells or every sixth grafted tissue section were incubated in primary antisera directed against TH overnight at 4°C. After Tris Buffer rinses, specimens were incubated in rat anti-mouse secondary antibody (Jackson, 1:200 with 1% goat serum) for 2 h, followed by rinses and finally incubated in Cy5 strepavidin tertiary antibody (Amersham, PA, Cy5 goat anti rat, 1:100) at room temperature for 1 h. Next, specimens were rinsed with Tris and incubated overnight at 4°C in primary antisera directed against β-galactosidase. After Tris Buffer rinses, specimens were incubated in goat anti rabbit secondary antibody (Jackson, 1:200, with 1% goat serum) for 2 h, rinsed and then incubated in Cy2 strepavidin tertiary antibody (Amersham, Cy2 goat anti-rabbit, 1:100). Sections were mounted on subbed slides, dehydrated to xylene and coverslipped in DPX mounting media. Sections and cells were examined using a Fluoview laser confocal system equipped with an Olympus microscope and argon/krypton lasers. Manual counts of THir, βgal-ir and double-labeled neurons within grafted tissue sections were made at 20X, the sum of these counts was then adjusted according to the method of Abercrombie [1]. Manual counts in cell culture were made in nine different center microisland fields at 20X as described previously [27, 40].
Statistical Analysis
Statistical analysis was performed using the Statview statistical package. ANOVA for repeated measures was used to analyze the data obtained from rotational assessment, while ANOVA was employed to analyze the data from all other measurements. The Bonferroni/Dunn post hoc test was used to determine significant differences between LDH levels, THir neurons, β-gal positive cells, and percentage of transduced THir neurons. The Fisher's Protected LSD post hoc test was used to determine significant differences between rotation rates, grafted THir neuron number and graftedß-gal-ir orß-gal-ir/THir cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 2.Anton R, Kordower JH, Kane DJ, Markham CH, Bredesen Neural transplantation of cells expressing the anti-apoptotic gene bcl-2. Cell Transplant. 1995;4:49–54. doi: 10.1177/096368979500400108. D.E. [DOI] [PubMed] [Google Scholar]
- 3.Bankiewicz KS, Leff SE, Nagy D, Jungles S, Rokovich J, Spratt K, Cohen L, Libonati M, Snyder RO, Mandel RJ. Practical aspects of the development of ex vivo and in vivo gene therapy for Parkinson's disease. Exp Neurol. 1997;144:147–156. doi: 10.1006/exnr.1996.6401. [DOI] [PubMed] [Google Scholar]
- 4.Barker RA, Dunnett SB, Faissner A, Fawcett JW. The time course of cell loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum. Exp Neurol. 1996;141:79–93. doi: 10.1006/exnr.1996.0141. [DOI] [PubMed] [Google Scholar]
- 5.Bowers WJ, Howard DF, Federoff HJ. Discordance between expression and genome transfer titering of HSV amplicon vectors: recommendation for standardized enumeration. Mol Ther. 2000;1(3):294–299. doi: 10.1006/mthe.2000.0039. [DOI] [PubMed] [Google Scholar]
- 6.Bowers WJ, Howard DF, Brooks AI, Halterman MW, Federoff HJ. Expression of vhs and VP16 during HSV-1 helper virus-free amplicon packaging enhances titers. Gene Ther. 2001;8(2):111–20. doi: 10.1038/sj.gt.3301340. [DOI] [PubMed] [Google Scholar]
- 7.Brewer GJ, Torricelli J, Evege EK, Price PJ. Neurobasal medium/B27 supplement: A new serum-free medium combination for survival of neurons. Focus. 1994;16:6–9. [Google Scholar]
- 8.Brewer GJ, Torricelli J, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–578. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 9.Brundin P, Strecker RE, Lindvall O, Isacson O, Nilsson OG, Bardin G, Prochiantz A, Forni C, Nieoullon A, Widner H, Gage FH, Bjorklund A. Intracerebral grafting of dopamine neurons. Annals NY Acad Sci. 1987;495:473–496. doi: 10.1111/j.1749-6632.1987.tb23695.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen DF, Schneider GE, Martinou JC, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385:434–439. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- 11.Counts SE, McGuire SO, Sortwell CE, Crawley JN, Collier TJ, Mufson EJ. Galanin inhibits tyrosine hydroxylase expression in midbrain dopaminergic neurons. J Neurochem. 2002;83(2):442–451. doi: 10.1046/j.1471-4159.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 12.Detrait ER, Bowers WJ, Halterman MW, Giuliano RE, Bennice L, Federoff HJ, Richfield EK. Reporter gene transfer induces apoptosis in primary cortical neurons. Mol Ther. 2002;5(6):723–30. doi: 10.1006/mthe.2002.0609. [DOI] [PubMed] [Google Scholar]
- 13.Duan WM, Widner H, Brundin P. Temporal pattern of host responses against intrastriatal grafts of syngeneic, allogeneic or xenogeneic embryonic neuronal tissue in rats. Exp Brain Res. 1995;104:227–242. doi: 10.1007/BF00242009. [DOI] [PubMed] [Google Scholar]
- 14.Emgard M, Karlsson J, Hansson O, Brundin P. Patterns of cell death and dopaminergic neuron survival in intrastriatal nigral grafts. Exp Neurol. 1999;160:279–288. doi: 10.1006/exnr.1999.7198. [DOI] [PubMed] [Google Scholar]
- 15.Fawcett JW, Barker RA, Dunnett SB. Dopaminergic neuronal survival and the effects of bFGF in explant, three dimensional and monolayer cultures of embryonic rat ventral mesencephalon. Exp Brain Res. 1995;106:275–282. doi: 10.1007/BF00241123. [DOI] [PubMed] [Google Scholar]
- 16.Fisher LJ, Jinnah HA, Kale LC, Higgins GA, Gage FH. Survival and function of intrastriatally grafted primary fibroblasts genetically modified to produce L-dopa. Neuron. 1991;6:371–380. doi: 10.1016/0896-6273(91)90246-v. [DOI] [PubMed] [Google Scholar]
- 17.Freed CR, Greene PE, Breeze RE, Tsai WY, Dumouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe parkinson's disease. N Engl J med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 18.Freed WJ, Geller HM, Poltorak M, Cannon-Spoor HE, Cottingham SL, Lamarca ME, Schultzberg M, Rehavi M, Paul S, Ginns EI. Genetically altered and defined cell lines for transplantation in animal models of Parkinson's disease. Prog Brain Res. 1990;82:11–21. doi: 10.1016/s0079-6123(08)62585-6. [DOI] [PubMed] [Google Scholar]
- 19.Geller AI, Breakefield XO. A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science. 1988;241(4873):1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geschwind MD, Kessler JA, Geller AI, Federoff HJ. Transfer of the nerve growth factor gene into cell lines and cultured neurons using a defective herpes simplex virus vector, Transfer of the NGF gene into cells by a HSV-1 vector. Mol Brain Res. 1994;24:327–335. doi: 10.1016/0169-328x(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 21.Holm KH, Cicchetti F, Bjorklund L, Boonman Z, Tandon P, Costantini LC, Deacon TW, Huang X, Chen DF, Isacson O. Enhanced axonal growth from fetal human bcl-2 transgenic mouse dopamine neurons transplanted to the adult rat striatum. Neurosci. 2001;104:397–405. doi: 10.1016/s0306-4522(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 22.Horellou P, Marlier L, Privat A, Darchen F, Scherman D, Henry JP, Mallet J. Exogenous expression of L-dopa and dopamine in various cell lines following transfer of rat and human tyrosine hydroxylase cDNA: Grafting in an animal model of Parkinson's disease. Prog Brain Res. 1990;82:23–32. doi: 10.1016/s0079-6123(08)62586-8. [DOI] [PubMed] [Google Scholar]
- 23.Jackson-Lewis V, Vila M, Djaldetti R, Guegan C, Liberatore G, Liu J, O'Malley KL, Burke RE, Przedborski S. Developmental cell death in dopaminergic neurons of the substantia nigra of mice. J Comp Neurol. 2000;424(3):476–488. doi: 10.1002/1096-9861(20000828)424:3<476::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Lu B, Federoff HJ. Herpes simplex virus type 1 amplicon vectors with glucocorticoid-inducible gene expression. Hum Gene Ther. 1995;6(4):419–28. doi: 10.1089/hum.1995.6.4-419. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg C, Horellou P, Mallet J, Bjorklund A. Generation of DOPA-producing astrocytes by retroviral transduction of the human tyrosine hydroxylase gene: In vitro characterization and in vivo effects in the rat Parkinson model. Exp Neurol. 1996;139:39–53. doi: 10.1006/exnr.1996.0079. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, Breeze R, Fahn S, Freed C, Eidelberg D. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann. Neurol. 2002;52(5):628–34. doi: 10.1002/ana.10359. [DOI] [PubMed] [Google Scholar]
- 27.Marchionini DM, Collier TJ, Camargo MD, McGuire SO, Pitzer MR, Sortwell CE. Interference with anoikis-induced cell death of dopamine neurons: Implications for augmenting embryonic graft survival in a rat model of Parkinson's disease. J. Comp. Neurol. 464(2):172–9. doi: 10.1002/cne.10785. [DOI] [PubMed] [Google Scholar]
- 28.Maries E, Kordower JH, Chu Y, Collier TJ, Sortwell CE, Olaru E, Shannon K, Steece-Collier K. Focal not widespread grafts induce novel dyskinetic behavior in parkinsonian rats. Neurobiol Dis. 2005 Aug 9; doi: 10.1016/j.nbd.2005.07.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Mouradian MM. Recent advances in the genetics and pathogenesis of Parkinson disease. Neurology. 2002;58(2):179–85. doi: 10.1212/wnl.58.2.179. [DOI] [PubMed] [Google Scholar]
- 30.Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann. Neurol. 2003;54(3):403–14. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 31.Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson's disease. TINS. 1996;19:102–109. doi: 10.1016/s0166-2236(96)80038-5. [DOI] [PubMed] [Google Scholar]
- 32.Olschowka JA, Bowers WJ, Hurley SD, Mastrangelo MA, Federoff HJ. Helper-free HSV-1 amplicons elicit a markedly less robust innate immune response in the CNS. Mol. Ther. 2003;7:218–227. doi: 10.1016/s1525-0016(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 33.O'Malley KL, Liu J, Lotharius J, Holtz W. Targeted expression of BCL-2 attenuates MPP+ but not 6-OHDA induced cell death in dopaminergic neurons. Neurobiol Dis. 2003;14(1):43–51. doi: 10.1016/s0969-9961(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 34.Paterson T, Everett RD. The regions of the herpes simplex virus type 1 immediate early protein Vmw175 required for site specific DNA binding closely correspond to those involved in transcriptional regulation. Nucleic Acids Res. 1988;16:11005–11025. doi: 10.1093/nar/16.23.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, Brundin P, Hagell P, Rehncrona S, Widner H, Lindvall O. Dopamine release from nigral transplants visualized in vivo in a parkinson's patient. Nat Neuro. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 36.Schierle GS, Leist M, Martinou JC, Widner H, Nicotera P, Brundin P. Differential effects of Bcl-2 overexpression on fibre outgrowth and survival of embryonic dopaminergic neurons in intracerebral transplants. Eur. J. Neurosci. 1999;11:3073–3081. doi: 10.1046/j.1460-9568.1999.00727.x. [DOI] [PubMed] [Google Scholar]
- 37.Sortwell CE. Strategies for the augmentation of grafted dopamine neuron survival. Front Biosci. 2003;8:s522–532. doi: 10.2741/1096. [DOI] [PubMed] [Google Scholar]
- 38.Sortwell CE, Camargo MD, Pitzer MR, Gyawali S, Collier TJ. Diminished survival of mesencephalic dopamine neurons grafted into aged hosts occurs during the immediate postgrafting interval. Exp Neurol. 2001;169(1):23–29. doi: 10.1006/exnr.2001.7644. [DOI] [PubMed] [Google Scholar]
- 39.Sortwell CE, Collier TJ, Camargo MD, Pitzer MR. An in vitro interval before transplantation of mesencephalic reaggregates does not compromise survival or functionality. Exp Neurol. 2004;187(1):58–64. doi: 10.1016/j.expneurol.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Sortwell CE, Daley BF, Pitzer MR, McGuire SO, Sladek JR, Jr, Collier TJ. Oligodendrocyte-type 2 astrocyte-derived trophic factors increase survival of developing dopamine neurons through the inhibition of apoptotic cell death. J Comp Neurol. 2000;426:143–153. [PubMed] [Google Scholar]
- 41.Sortwell CE, Pitzer MR, Collier TJ. Time course of apoptotic cell death within mesencephalic cell suspension grafts: Implications for improving grafted dopamine neuron survival. Exp Neurol. 2000;165:268–277. doi: 10.1006/exnr.2000.7476. [DOI] [PubMed] [Google Scholar]
- 42.Sortwell CE, Kordower JH. In vivo gene therapy as a potential treatment for Parkinsons disease. In: Olanow W, Brundin P, editors. Restorative Therapies in Parkinsons Disease. 2006. pp. 317–344. [Google Scholar]
- 43.Stavropoulos TA, Strathdee CA. An enhanced packaging system for helper-dependent herpes simplex virus vectors. J. Virol. 1998;72:7137–7143. doi: 10.1128/jvi.72.9.7137-7143.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steece-Collier K, Collier TJ, Danielson PD, Kurlan R, Yurek DM, Sladek JR., Jr. Embryonic mesencephalic grafts increase levodopa-induced forelimb hyperkinesia in parkinsonian rats. Mov Disord. 2003;18(12):1442–54. doi: 10.1002/mds.10588. [DOI] [PubMed] [Google Scholar]
- 45.Takeshima T, Shimoda K, Johnston JM, Commissiong JW. Standardized methods to bioassay neurotrophic factors for dopaminergic neurons. J Neurosci Meth. 1996;67:27–41. [PubMed] [Google Scholar]
- 46.Tsai DF, Ho JJ, Ozawa CR, Sapolsky RM. Long-term expression driven by herpes simplex virus type-1 amplicons may fail due to eventual degradation or extrusion of introduced transgenes. Exp Neurol. 2000;165(1):58–65. doi: 10.1006/exnr.2000.7454. [DOI] [PubMed] [Google Scholar]
- 47.Tseng JL, Baetge EE, Zurn AD, Aebischer P. GDNF reduces drug-induced rotational behavior after medial forebrain bundle transection by a mechanism not involving striatal dopamine. J. Neurosci. 1997;17:325–333. doi: 10.1523/JNEUROSCI.17-01-00325.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Yu L, Geller AI. Diverse stabilities of expression in the rat brain from different cellular promoters in a helper virus-free herpes simplex virus type 1 vector system. Hum Gene Ther. 1999;10(11):1763–71. doi: 10.1089/10430349950017446. [DOI] [PubMed] [Google Scholar]
- 49.Wolff JA, Fisher LJ, Xu L, Jinnah HA, Langlais PJ, Iuvone PM, O'Malley KL, Rosenberg MB, Shimohama S, Friedmann T. Grafting fibroblasts genetically modified to produce L-dopa in a rat model of Parkinson disease. Proc Natl Acad Sci U. S. A. 1989;86:9011–9014. doi: 10.1073/pnas.86.22.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yurek DM, Sladek JR., Jr. Dopamine cell replacement: Parkinson's disease. Ann Rev Neurosci. 1990;13:415–440. doi: 10.1146/annurev.ne.13.030190.002215. [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92(1):57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]


