Abstract
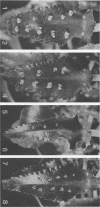
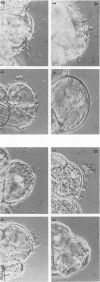
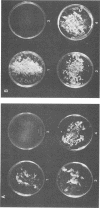
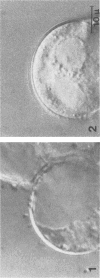
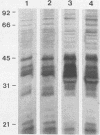
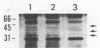
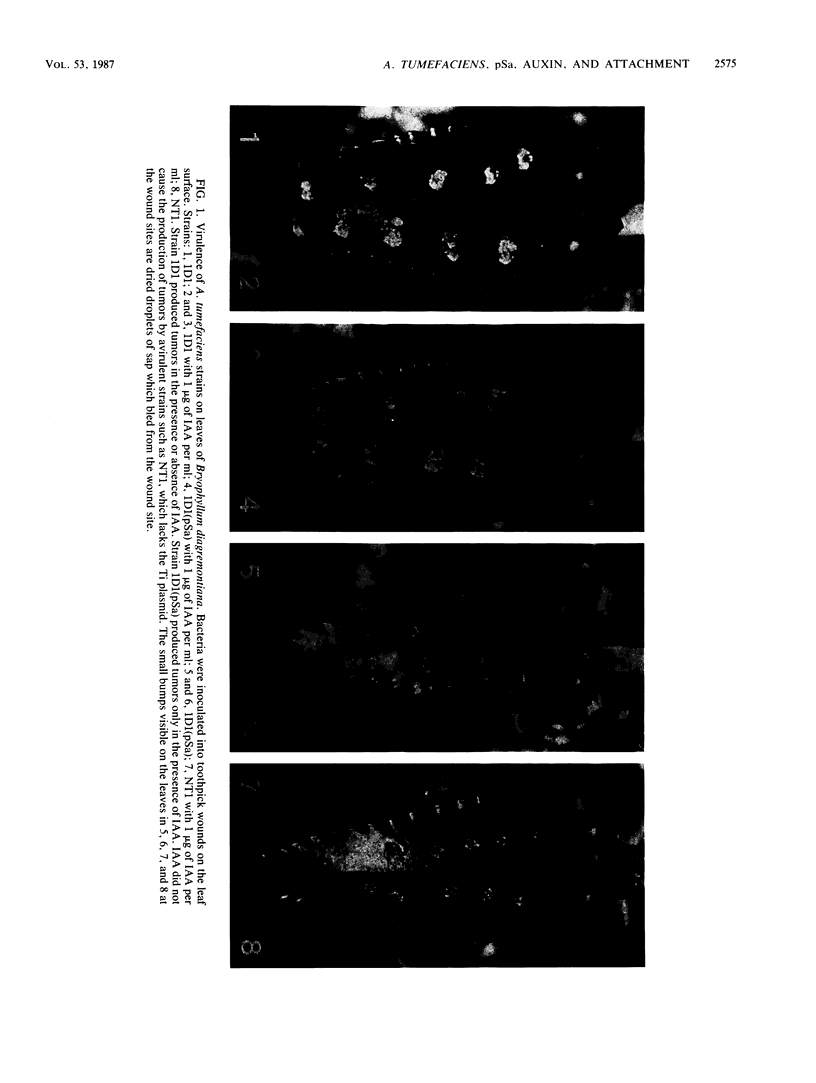
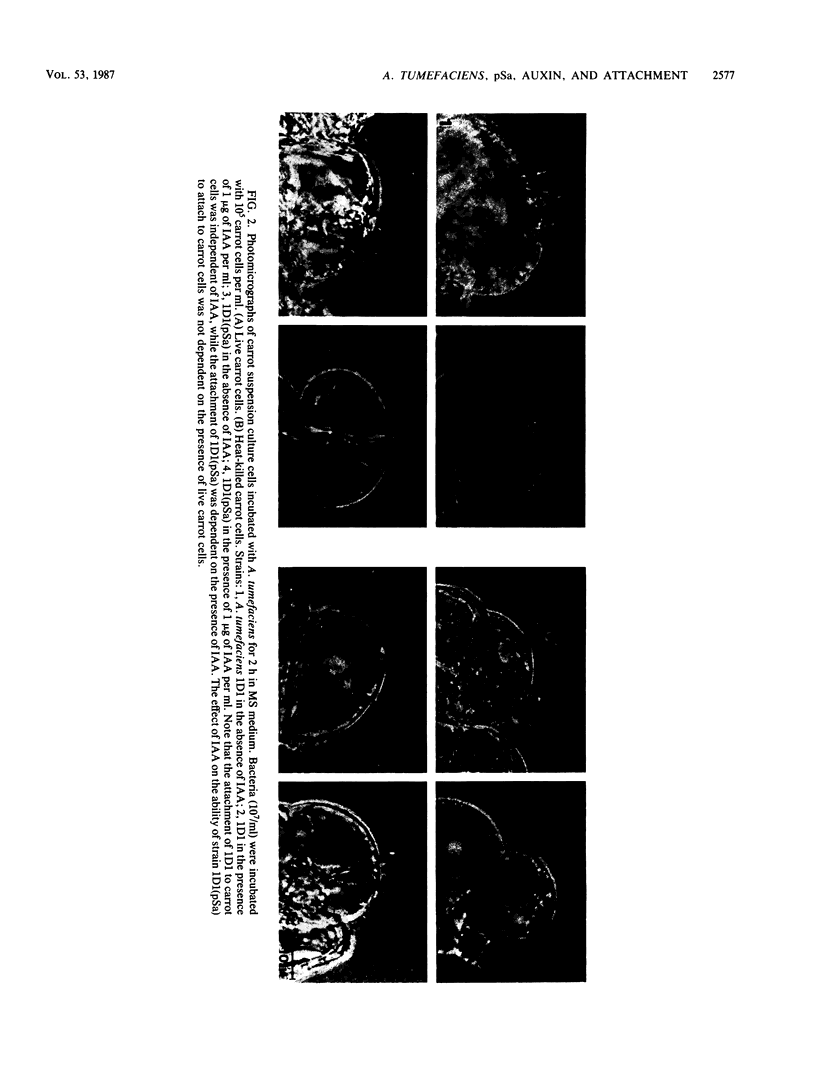
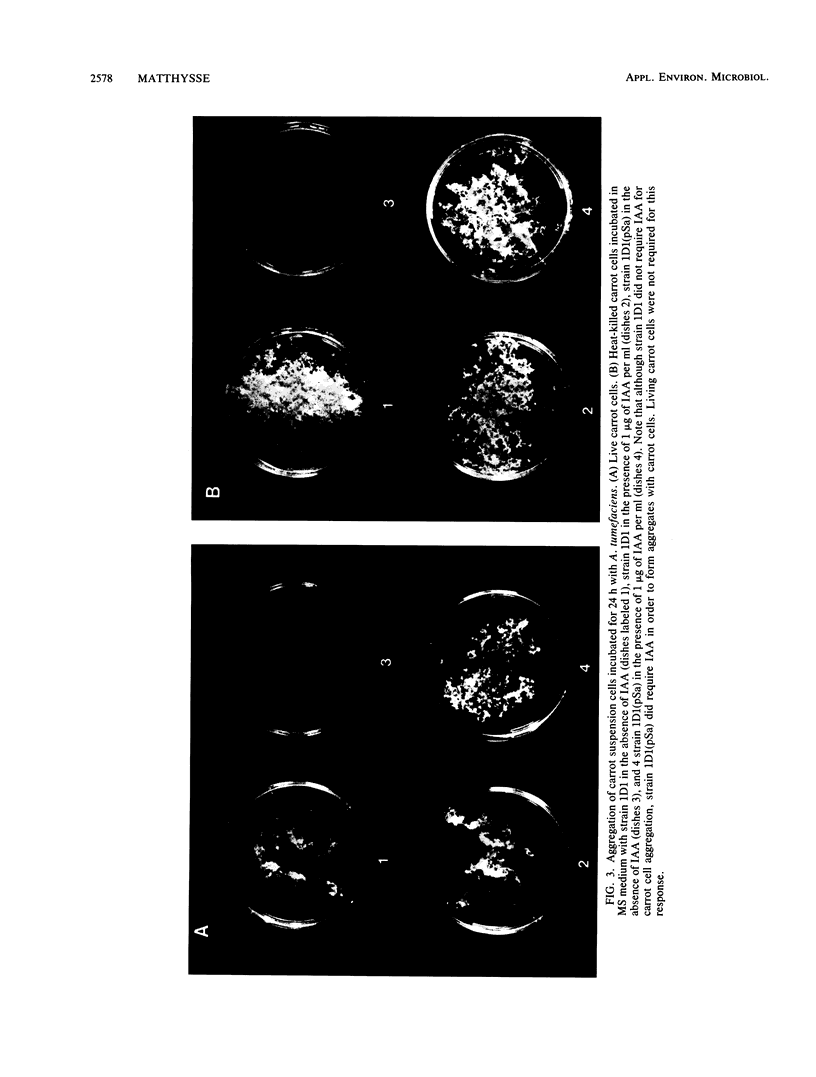
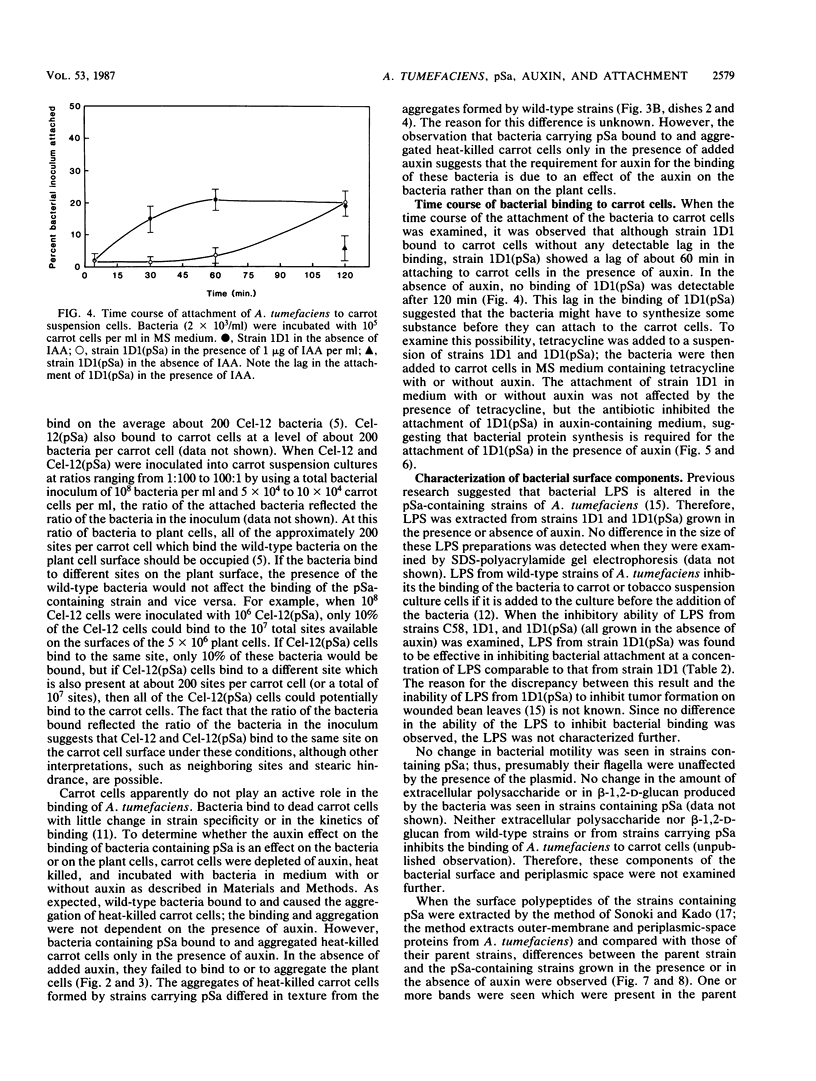
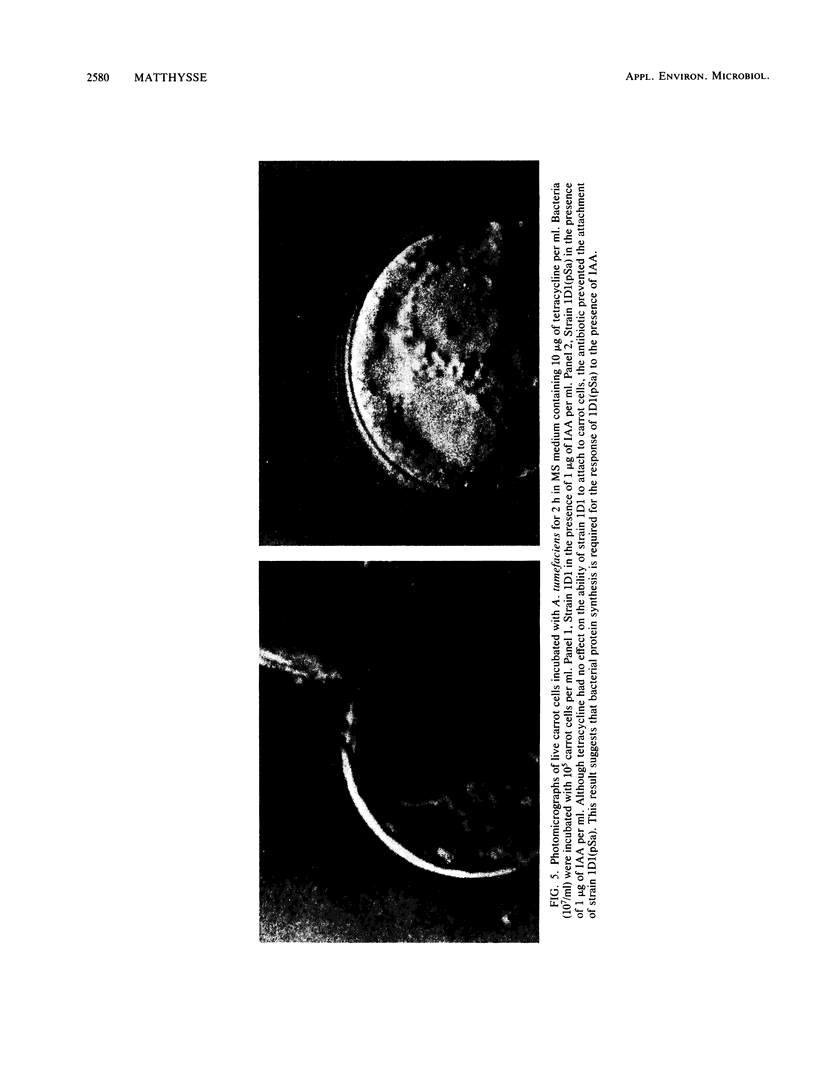
When the plasmid pSa is introduced into Agrobacterium tumefaciens, its presence results in the suppression of bacterial virulence. A. tumefaciens(pSa) cells are virulent on Bryophyllum diagremontiana only when inoculated with auxin. A. tumefaciens(pSa) cells also bind to plant cells only in the presence of auxin. The effect of auxin is on the bacteria rather than on the plant cells, since the bacteria require auxin to bind to heat-killed carrot cells. Bacteria containing pSa and grown in the absence of auxin showed a lag in binding to carrot cells in auxin-containing medium. This lag was not seen during the binding of wild-type strains. Tetracycline inhibited the binding of A. tumefaciens(pSa) in auxin-containing medium, suggesting that bacterial protein synthesis is required for the auxin effect. No difference was seen in the size or ability to inhibit bacterial binding of lipopolysaccharides from bacteria containing or lacking pSa and grown with or without auxin. A. tumefaciens(pSa) cells grown in the absence of auxin lacked surface polypeptide(s) found in bacteria grown in the presence of auxin and in the wild-type bacteria, which do not contain pSa. Thus, the presence of certain polypeptides appears to be associated with the ability of the bacteria to bind to plant cells.
Full text
PDF
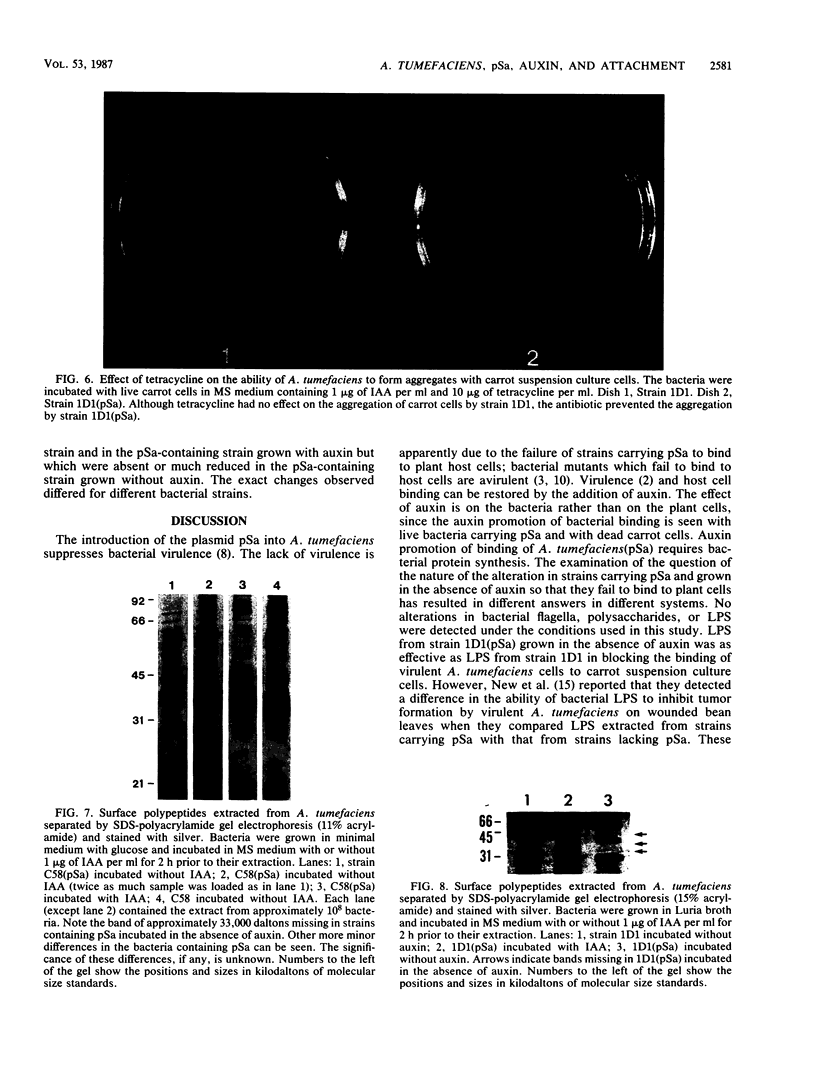

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlson R. W., Lee R. P. A Comparison of the Surface Polysaccharides from Rhizobium leguminosarum 128C53 smrif with the Surface Polysaccharides from Its Exo Mutant. Plant Physiol. 1983 Feb;71(2):223–228. doi: 10.1104/pp.71.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Halperin W., Nester E. W. Agrobacterium tumefaciens mutants affected in attachment to plant cells. J Bacteriol. 1982 Dec;152(3):1265–1275. doi: 10.1128/jb.152.3.1265-1275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurlitz R. H., Lamb P. W., Matthysse A. G. Involvement of Carrot Cell Surface Proteins in Attachment of Agrobacterium tumefaciens. Plant Physiol. 1987 Mar;83(3):564–568. doi: 10.1104/pp.83.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lippincott B. B., Lippincott J. A. Bacterial attachment to a specific wound site as an essential stage in tumor initiation by Agrobacterium tumefaciens. J Bacteriol. 1969 Feb;97(2):620–628. doi: 10.1128/jb.97.2.620-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loper J. E., Kado C. I. Host range conferred by the virulence-specifying plasmid of Agrobacterium tumefaciens. J Bacteriol. 1979 Aug;139(2):591–596. doi: 10.1128/jb.139.2.591-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G. Characterization of nonattaching mutants of Agrobacterium tumefaciens. J Bacteriol. 1987 Jan;169(1):313–323. doi: 10.1128/jb.169.1.313-323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G., Holmes K. V., Gurlitz R. H. Elaboration of cellulose fibrils by Agrobacterium tumefaciens during attachment to carrot cells. J Bacteriol. 1981 Jan;145(1):583–595. doi: 10.1128/jb.145.1.583-595.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J Bacteriol. 1983 May;154(2):906–915. doi: 10.1128/jb.154.2.906-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G., Wyman P. M., Holmes K. V. Plasmid-dependent attachment of Agrobacterium tumefaciens to plant tissue culture cells. Infect Immun. 1978 Nov;22(2):516–522. doi: 10.1128/iai.22.2.516-522.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Stacey G., Douglas C. J., Nester E. W. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985 Oct;164(1):102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoki S., Kado C. I. Proteins conferred by the virulence-specifying plasmid of Agrobacterium tumefaciens C-58. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3796–3800. doi: 10.1073/pnas.75.8.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]