Abstract
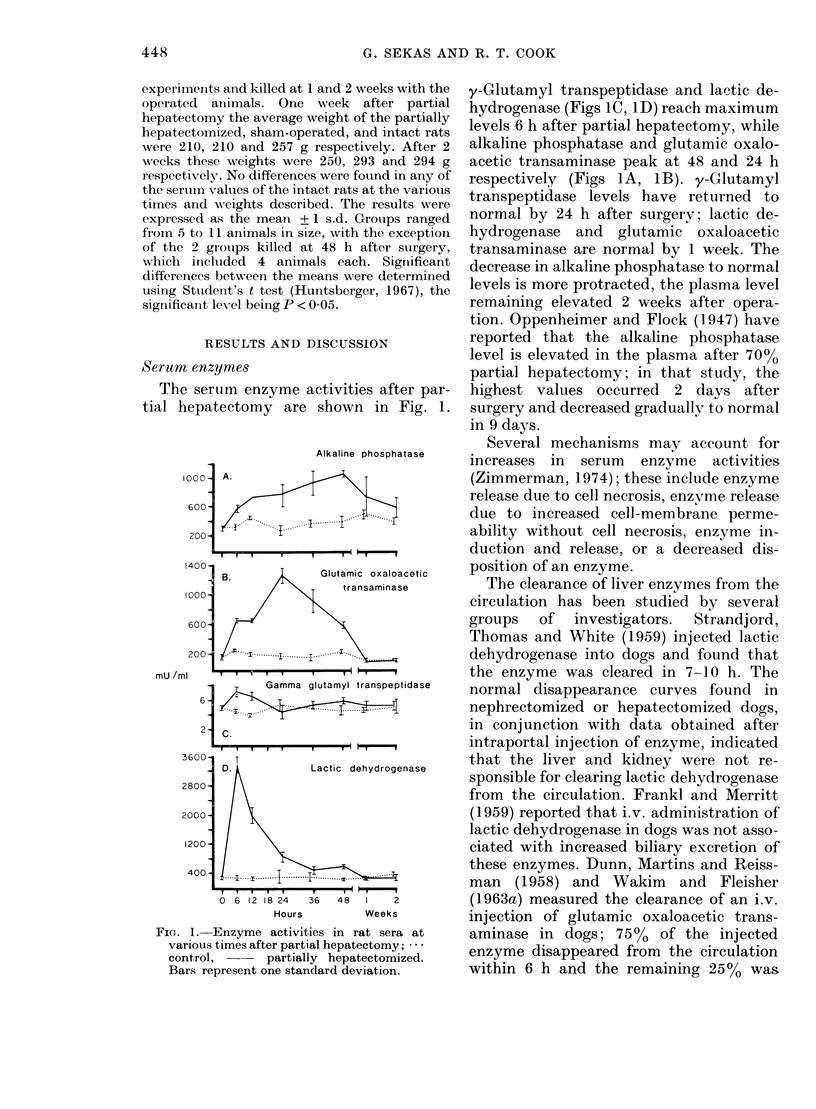
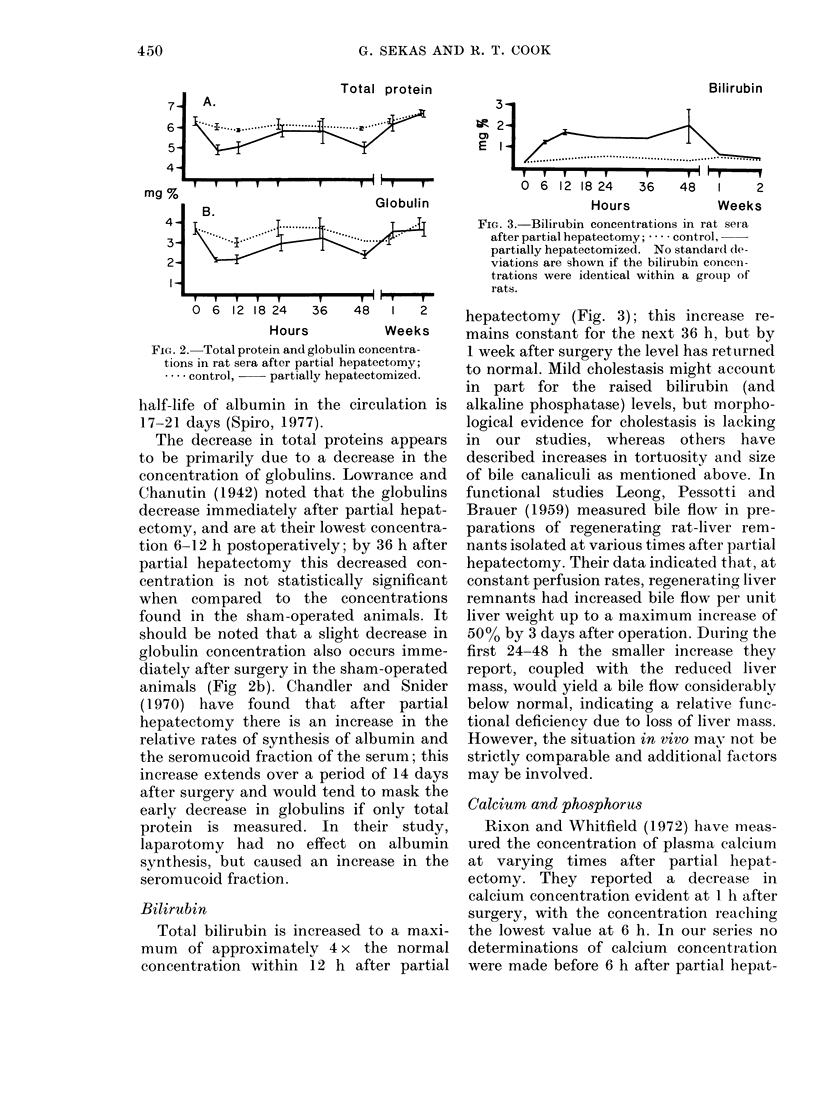
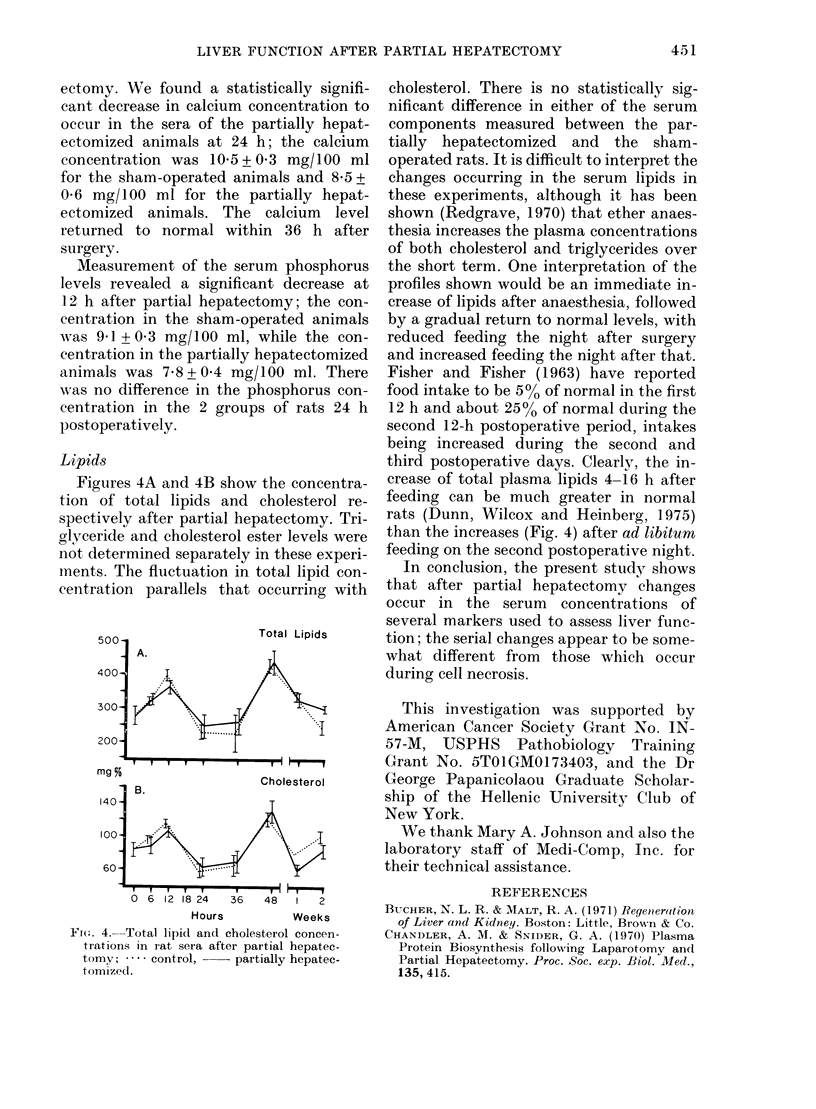
In serial studies of hepatic function in rats after 70% partial hepatectomy, quantitative changes were found in several of the serum components used clinically to assess liver status. The activities of the following enzymes were found to increase: gamma-glutamyl transpeptidase and lactic dehydrogenase were maximal 6 h postoperatively, while glutamic oxaloacetic transaminase and alkaline phosphatase reached peak values at 24 and 48 h respectively. Albumin levels were found to be relatively constant during the study; however, total protein concentration was lowest 6--12 h postoperatively, paralleling a decrease in globulin concentration. Bilirubin levels were elevated to 4x normal within 12 h after surgery. After partial hepatectomy calcium and phosphorus concentrations were significantly decreased at 24 and 12 h respectively. With the exception of alkaline phosphatase, the activities of all serum components measured returned to normal levels by 1 week after surgery; the alkaline phosphatase concentration continued to be elevated 2 weeks postoperatively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler A. M., Snider G. A. Plasma protein biosynthesis following laparotomy and partial hepatectomy. Proc Soc Exp Biol Med. 1970 Nov;135(2):415–418. doi: 10.3181/00379727-135-35064. [DOI] [PubMed] [Google Scholar]
- Curtis S. J., Moritz M., Snodgrass P. J. Serum enzymes derived from liver cell fractions. I. The response to carbon tetrachloride intoxication in rats. Gastroenterology. 1972 Jan;62(1):84–92. [PubMed] [Google Scholar]
- DUNN M., MARTINS J., REISSMANN K. R. The disappearance rate of glutamic oxalacetic transaminase from the circulation and its distribution in the body's fluid compartments and secretions. J Lab Clin Med. 1958 Feb;51(2):259–265. [PubMed] [Google Scholar]
- Dunn G. D., Wilcox H. G., Heimberg M. Temporal relationships between dietary, plasma, hepatic, and adipose tissue lipids after short-term feeding of safflower oil to rats. J Lab Clin Med. 1975 Sep;86(3):369–377. [PubMed] [Google Scholar]
- FISHER E. R., FISHER B. ULTRASTRUCTURAL HEPATIC CHANGES FOLLOWING PARTIAL HEPATECTOMY AND PORTACAVAL SHUNT IN THE RAT. Lab Invest. 1963 Sep;12:929–942. [PubMed] [Google Scholar]
- FLEISHER G. A., WAKIM K. G. The fate of enzymes in body fluids-an experimental study. I. Disappearance rates of glutamic-pyruvic transaminase under various conditions. J Lab Clin Med. 1963 Jan;61:76–85. [PubMed] [Google Scholar]
- FRANKL H. D., MERRITT J. H. Enzyme activity in the serum and common duct bile of dogs. Am J Gastroenterol. 1959 Feb;31(2):166–170. [PubMed] [Google Scholar]
- Kaplan M. M., Righetti A. Induction of rat liver alkaline phosphatase: the mechanism of the serum elevation in bile duct obstruction. J Clin Invest. 1970 Mar;49(3):508–516. doi: 10.1172/JCI106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES K. R., SINHA K. P. Blood enzymes in liver injury. J Pathol Bacteriol. 1960 Oct;80:297–307. [PubMed] [Google Scholar]
- Redgrave T. G. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970 Mar;49(3):465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon R. H., Whitfield J. F. Parathyroid hormone: a possible initiator of liver regeneration. Proc Soc Exp Biol Med. 1972 Oct;141(1):93–97. doi: 10.3181/00379727-141-36723. [DOI] [PubMed] [Google Scholar]
- STRANDJORD P. E., THOMAS K. E., WHITE L. P. Studies on isocitric and lactic dehydrogenases in experimental myocardial infarction. J Clin Invest. 1959 Dec;38:2111–2118. doi: 10.1172/JCI103989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczuk A. A soluble form of gamma-glutamyl transpeptidase in human tissues. Clin Chim Acta. 1966 Nov;14(5):608–614. doi: 10.1016/0009-8981(66)90184-7. [DOI] [PubMed] [Google Scholar]
- WAKIM K. G., FLEISHER G. A. The fate of enzymes in body fluids--an experimental study. II. Disappearance rates of glutamic-oxalacetic transaminase I under various conditions. J Lab Clin Med. 1963 Jan;61:86–97. [PubMed] [Google Scholar]


